睾丸融合的显微外科梗阻
Summary
将铝箔显微外科插入 Spodoptera litura 的睾丸之间,以阻碍睾丸的融合。该过程包括冷冻,固定,消毒,切口,放置屏障,缝合,术后喂养和检查。这种方法提供了一种干扰组织形成的方法。
Abstract
没有使用RNA干扰(RNAi)和簇状规则间隔的短回文重复序列(CRISPR)/ CRISPR相关的核酸内切酶Cas9等遗传方法,而是在 Spodoptera litura 的睾丸之间插入物理屏障,以研究这种显微手术对其生长和繁殖的影响。在睾丸之间插入铝箔后,昆虫在变质过程中的蜕皮正常进行。昆虫的生长和发育没有显着改变;然而,如果显微外科手术停止睾丸融合,精子束的数量就会发生变化。这些发现表明,阻断睾丸融合会影响男性生殖能力。该方法可进一步应用于中断器官之间的通信,以研究特定信号通路的功能。与传统手术相比,显微外科手术只需要冷冻麻醉,比二氧化碳麻醉更可取。显微外科手术还可以最大限度地减少手术部位区域并促进伤口愈合。但是,具有特定功能的材料的选择需要进一步研究。在手术过程中做切口时,避免组织损伤至关重要。
Introduction
融合是组织或器官发育中的常见现象。例子包括 果蝇1中的背侧闭合和胸部闭合以及小鼠和鸡的腭形态发生,神经管形态发生以及心脏形态发生2。CRISPR和RNAi已被应用于研究基因在融合过程中的作用2,3,4。
Spodoptera litura(S. litura,鳞翅目: Noctuidae)是一种有害的多食性害虫,广泛分布在亚洲的热带和亚热带地区,包括中国4,5,6。 S. litura 的广泛分布部分归因于其强大的生殖能力,这与性腺发育有关。男性不育症是控制这种害虫的一种方法。如睾丸结构示意图所示,睾丸被睾丸鞘包围,包括外鞘(腹膜鞘)和内基底层。基底层向内延伸以形成滤泡上皮,并将睾丸的内部区域分成四个称为卵泡的腔室(图1)。
在卵泡中,精子形成有丝分裂和减数分裂后发育成精子,然后精子囊中的精子朝同一方向排列形成精子束7。在精子发生过程中,初级精子细胞分化为真芘精子或芘精子。幼虫期的精子细胞发育成真芘精子,长尾巴连接到细长的细胞核的头部;这些可以使卵子受精。相反,蛹中期的精子细胞发育成具有废弃细胞核的芘精子;这些精子有助于芘啉精子的存活、运动和受精9,10。蛹的第 6天是睾丸具有丰富的真芘和芘精子束的时期。
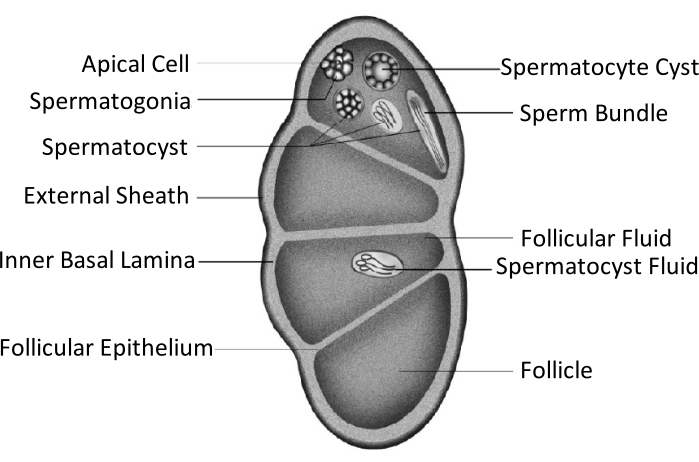
图 1:鳞翅目昆虫睾丸结构示意图11。请单击此处查看此图的放大版本。
睾丸融合发生在鳞翅目的大多数昆虫11,12中,特别是在那些农业害虫中。睾丸融合是指一对睾丸在幼虫期双侧生长,彼此接近并粘附,最终整合成单个性腺11。在 Spodoptera litura 中 ,它发生在从幼虫到蛹阶段的过程中。从5龄(L5D1)的第 1天到6龄(L6D4)的第 4天,这对睾丸的大小逐渐增长,颜色从象牙白色变为浅黄色。当它到达前期(L6D5至L6D6)时,它会变成淡红色。两个双侧对称睾丸在前裆阶段相互接近,融合为一个,逆时针扭曲(多拉视图),在蛹期和成虫期产生单个睾丸11。这种现象不会发生在蚕身上,蚕具有相当大的经济重要性,并且已经驯化了5000年13。因此,假设睾丸的融合提高了生殖能力。
为了确定 踞 踤荠睾融合术的意义,重要的是要研究阻断该过程的影响。在该协议中,在睾丸之间微外科插入铝箔以保持它们分离,并研究了昆虫及其睾丸发育的相应变化。
Protocol
Representative Results
Discussion
在 对Spodoptera litura的睾丸融合进行显微外科手术后,精子束的数量减少,这支持了这种融合有利于生殖能力的假设。自20世纪初 以来,外科手术一直被用于研究昆虫的生理发育。为了确定颅神经是否受到昆虫的调节,一些研究人员对不同的昆虫(包括半翅目 Rhodnius prolixus ,鳞翅目中的 Lymantria dispar )进行了结扎和斩首等程序 15 , <sup class="xre…
Declarações
The authors have nothing to disclose.
Acknowledgements
这项工作得到了国家自然科学基金(Nos.:31772519,31720103916;)和西南大学蚕基因组生物学国家重点实验室(编号:sklsgb2013003)的公开资助。
Materials
| 75% Rubbing alcohol | Qingdao Hainuo Nuowei Disinfection Technology Co., Ltd | Q/370285HNW 001-2019 | |
| Colored Push Pins | Deli Group Co.,LTD | 0042 | |
| Corneal Scissors | Suzhou Xiehe Medical Device Co., Ltd | MR-S221A | Curved and blunt tip |
| Glad Aluminum Foil | Clorox China(Guangzhou) Limited | 831457 | 10 cm*2.5 cm*0.6 |
| Medical Cotton Swabs (Sterile) | Winner Medical Co., Ltd. | 601-022921-01 | |
| Medical Iodine Cotton Swab | Winner Medical Co., Ltd. | 608-000247 | |
| Needle holder | Shanghai Medical Instruments (Group) Ltd., Corp. | J32030 | 14 cm fine needle |
| Sterile surgical blade | Shanghai Pudong Jinhuan Medical Supplies Co., LTD | #11 | |
| Suigical Blade Holder | Shanghai Pudong Jinhuan Medical Supplies Co., LTD | K6-10 | Straight 3# |
| Suture thread with needle | Ningbo Medical Stitch Needle Co., Ltd | needle: 3/8 Circle, 2.5*8 ; Thread: Nylon, 6/0, 25 cm | |
| Tying Forceps | Suzhou Xiehe Medical Device Co., Ltd | MR-F201T-3 | Straight-pointed; long handle; 0.12 mm-wide-head |
Referências
- Zeitlinger, J., Bohmann, D. Thorax closure in Drosophila: involvement of Fos and the JNK pathway. Development. 126 (17), 3947-3956 (1999).
- Ray, H. J., Niswander, L. Mechanisms of tissue fusion during development. Development. 139 (10), 1701-1711 (2012).
- Ducuing, A., Keeley, C., Mollereau, B., Vincent, S. A. DPP-mediated feed-forward loop canalizes morphogenesis during Drosophila dorsal closure. The Journal of Cell Biology. 208 (2), 239-248 (2015).
- Du, Q., et al. Identification and functional characterization of doublesex gene in the testis of Spodoptera litura. Insect Science. 26 (6), 1000-1010 (2019).
- Qin, H. G., Ding, J., Ye, Z. X., Huang, S. J., Luo, R. H. Dynamic analysis of experimental population of Spodoptera litura. Journal of Biosafety. 13 (2), 45-48 (2004).
- Guan, B. B., et al. The research in biology and ecology of Spodoptera litura. Journal of Biosafety. 8 (1), 57-61 (1999).
- Wen, L., et al. The testis development and spermatogenesis in Spodopture litura (lepidoptera: noctuidae). Journal of South China Normal University (Natural Science Edition. 51 (4), 47-56 (2019).
- Friedländer, M., Seth, R. K., Reynolds, S. E. Eupyrene and apyrene sperm: dichotomous spermatogenesis in Lepidoptera. Advances in Insect Physiology. 32, 206 (2010).
- Cook, P. A., Wedell, N. Non-fertile sperm delay female remating. Nature. 397 (6719), 486 (1999).
- Iriki, S. On the function of apyrene spermatozoa in the silk worm. Zoological Magazine. 53, 123-124 (1941).
- Liu, L., Feng, Q. L. The study of fusion of testis in Lepidoptera insects. Journal of South China Normal University (Natural Science Edition). 46 (5), 1-7 (2014).
- Klowden, M. J. . Physiological systems in insects. , (2007).
- Xu, J., et al. Transgenic characterization of two testis-specific promoters in the silkworm, Bombyx mori. Insect Molecular Biology. 24 (2), 183-190 (2015).
- Guo, X. R., Zheng, S. C., Liu, L., Feng, Q. L. The sterol carrier protein 2/3-oxoacyl-CoA thiolase (SCPx) is involved in cholesterol uptake in the midgut of Spodoptera litura: gene cloning, expression, localization and functional analyses. BioMed Central Molecular Biology. 10, 102 (2009).
- Kopeć, S. Studies on the necessity of the brain for the inception of insect metamorphosis. Biological Bulletin. 42 (6), 323-342 (1922).
- Wigglesworth, V. B. Factors controlling moulting and ‘metamorphosis’ in an insect. Nature. 133 (5), 725-726 (1934).
- Williams, C. N. Physiology of insect diapause; the role of the brain in the production and termination of pupal dormancy in the giant silkworm, Platysamia cecropia. Biological Bulletin. 90 (3), 234-243 (1946).
- Fukuda, S. Hormonal control of molting and pupation in the silkworm. Proceedings of the Imperial Academy Tokyo. 16 (8), 417-420 (1940).
- Tian, H. J., Liu, Z. P., Bai, Y. Y. Common methods to detect Sperm quality of mammalian. Journal of Economic Zoology. 8 (4), 198-201 (2004).
- Ji, X. S., Chen, S. L., Zhao, Y., Tian, Y. S. Progress in the quality evaluation of fish sperm. Chinese Fishery Science. 14 (6), 1048-1054 (2007).
- Baulny, B. O. D., Vern, Y. L., Kerboeuf, D., Maisse, G. Flow cytometric evaluation of mitochondrial activity and membrane integrity in fresh and cryopreserved rainbow trout (Oncorhynchus mykiss) spermatozoa. Cryobiology. 34 (2), 141-149 (1997).
- Krasznai, Z., Márián, T., Balkay, I., Emri, M., Trón, L. Permeabilization and structural changes in the membrane of common carp (Cyprinus carpio L.) sperm induced by hypo-osmotic shock. Aquaculture. 129 (1), 134 (1995).
- Kime, D. E., et al. Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comparative Biochemistry & Physiology Toxicology & Pharmacology Cbp. 130 (4), 425-433 (2001).
- Rurangwa, E., Volckaert, F. A., Huyskens, G., Kime, D. E., Ollevier, F. Quality control of refrigerated and cryopreserved semen using computer-assisted sperm analysis (CASA), viable staining and standardized fertilization in African catfish (Clarias gariepinus). Theriogenology. 55 (3), 751-769 (2001).
- Seth, R. K., Kaur, J. J., Rao, D. K., Reynolds, S. E. Effects of larval exposure to sublethal concentrations of the ecdysteroid agonists RH-5849 and tebufenozide (RH-5992) on male reproductive physiology in Spodoptera litura. Journal of Insect Physiology. 50 (6), 505-517 (2004).
- Sweeney, R. M., Watterson, R. L. Rib development in chick embryos analyzed by means of tantalum foil blocks. American Journal of Anatomy. 126 (2), 127-149 (1969).
- Wilde, S., Logan, M. P. Application of impermeable barriers combined with candidate factor soaked beads to study inductive signals in the chick. Journal of Visualized Experiments. (117), e54618 (2016).

