使用生物表面活性剂组合提高石油采收率
Summary
我们说明了筛选和鉴定产生生物表面活性剂的微生物的方法。还介绍了生物表面活性剂的色谱表征和化学鉴定方法,确定了生物表面活性剂在提高残油采收率方面的工业适用性。
Abstract
生物表面活性剂是表面活性化合物,能够降低两个不同极性的相之间的表面张力。生物表面活性剂已成为化学表面活性剂的有前途的替代品,因为它具有较低的毒性,高生物降解性,环境相容性和对极端环境条件的耐受性。在这里,我们说明了用于筛选能够产生生物表面活性剂的微生物的方法。使用滴落塌陷,油扩散和乳液指数测定来鉴定产生生物表面活性剂的微生物。通过确定由于微生物构件的生长而导致的介质表面张力的降低,验证了生物表面活性剂的生产。我们还描述了生物表面活性剂表征和鉴定所涉及的方法。对提取的生物表面活性剂进行薄层色谱,然后对板进行差异染色以确定生物表面活性剂的性质。LCMS, 1H NMR和FT-IR用于化学鉴定生物表面活性剂。我们进一步说明了评估所生产生物表面活性剂组合在模拟砂堆积柱中提高残余油采收率的方法。
Introduction
生物表面活性剂是由微生物产生的两亲表面活性分子,具有降低表面和两相1之间的界面张力的能力。典型的生物表面活性剂含有亲水部分,其通常由糖部分或肽链或亲水氨基酸组成,疏水部分由饱和或不饱和脂肪酸链2组成。由于其两亲性质,生物表面活性剂在两相之间的界面处组装并降低边界处的界面张力,这有助于一相分散到另一相1,3中。迄今为止报道的各种类型的生物表面活性剂包括糖脂,其中碳水化合物 通过 酯键(例如,鼠李脂,三卤脂和桔梗脂)与长链脂肪族或羟基脂肪族酸相连,脂质附着在多肽链上的脂肽(例如,表面活性素和地衣官能素),以及通常由多糖 – 蛋白质复合物组成的聚合物生物表面活性剂(例如, 乳聚糖,脂质,阿拉桑和脂甘露聚糖)4。由微生物产生的其他类型的生物表面活性剂包括脂肪酸、磷脂、中性脂质和颗粒生物表面活性剂5.研究最多的一类生物表面活性剂是糖脂类,其中大多数研究都报告了鼠李糖脂6。鼠李糖含有一个或两个鼠李糖分子(形成亲水部分),与一个或两个长链脂肪酸分子(通常是羟基癸酸)相连。鼠李糖脂是首先从 铜绿假单胞菌7 中报告的原代糖脂。
与化学表面活性剂相比,生物表面活性剂因其提供的各种独特和独特的特性而受到越来越多的关注8.这些包括更高的特异性,更低的毒性,更大的多样性,易于制备,更高的生物降解性,更好的发泡性,环境相容性和极端条件下的活性9。生物表面活性剂的结构多样性(图S1)是另一个优点,使它们比化学对应物10更具优势。它们在较低浓度下通常更有效和高效,因为它们的临界胶束浓度(CMC)通常比化学表面活性剂11低几倍。据报道,它们具有高度耐热性(高达100°C),并且可以耐受更高的pH值(高达9)和高盐浓度(高达50 g / L)12 因此在需要暴露在极端条件下的工业过程中具有多种优势13。生物降解性和较低的毒性使它们适用于环境应用,如生物修复。由于它们提供的优势,它们在食品,农业,洗涤剂,化妆品和石油工业等各个行业中得到了越来越多的关注11.生物表面活性剂在石油修复中也引起了很多关注,以去除石油污染物和有毒污染物14。
在这里,我们报告了由 红球菌 IITD102, Lysinibacillus sp. IITD104和 Paenibacillus sp. IITD108生产的生物表面活性剂的生产,表征和应用。 图1概述了筛选,表征和应用生物表面活性剂组合以提高石油采收率所涉及的步骤。
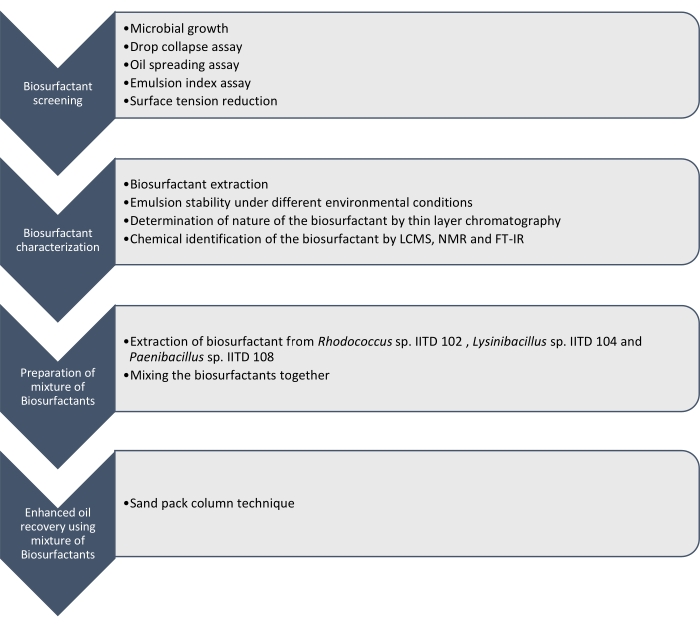
图1:使用生物表面活性剂组合提高石油采收率的方法。 图中显示了逐步工作流程。这项工作分四个步骤进行。首先,通过各种测定对微生物菌株进行培养和筛选,以生产生物表面活性剂,包括滴坍试验,油扩散测定,乳化指数测定和表面张力测量。然后,从无细胞肉汤中提取生物表面活性剂,并使用薄层色谱法鉴定其性质,并使用LCMS,NMR和FT-IR进一步鉴定它们。在下一步中,将提取的生物表面活性剂混合在一起,并使用砂包柱技术确定所得混合物提高石油采收率的潜力。 请点击此处查看此图的大图。
通过滴坍,油铺,乳液指数测定和测定由于微生物生长引起的无细胞培养基表面张力的降低,对这些微生物菌株进行筛选以产生生物表面活性剂。通过LCMS, 1H NMR和FT-IR提取,表征和化学鉴定生物表面活性剂。最后,制备了这些微生物产生的生物表面活性剂混合物,并用于回收模拟砂包柱中的残余油。
本研究仅说明了生物表面活性剂组合在提高残油采收率方面的筛选、鉴定、结构表征和应用方法。它没有提供由微生物菌株15,16产生的生物表面活性剂的详细功能表征。执行各种实验,例如关键胶束测定,热重分析,表面润湿性和生物降解性,以对任何生物表面活性剂进行详细的功能表征。但由于本文是方法论文,因此重点在于生物表面活性剂组合在提高残油采收率方面的筛选、鉴定、结构表征和应用;这些实验尚未纳入本研究。
Protocol
Representative Results
Discussion
生物表面活性剂是最通用的生物活性成分之一,正在成为化学表面活性剂的有吸引力的替代品。它们具有更好的润湿性,较低的CMC,多样化的结构和环保性,因此在洗涤剂,油漆,化妆品,食品,制药,农业,石油和水处理等众多行业中具有广泛的应用18.这导致人们越来越有兴趣发现更多能够生产生物表面活性剂的微生物菌株。在这里,我们说明了筛选,鉴定和应用由红球菌…
Declarações
The authors have nothing to disclose.
Acknowledgements
作者要感谢印度政府生物技术部的财政支持。
Materials
| 1 ml pipette | Eppendorf, Germany | G54412G | |
| 1H NMR | Bruker Avance AV-III type spectrometer,USA | ||
| 20 ul pipette | Thermo scientific, USA | H69820 | |
| Autoclave | JAISBO, India | Ser no 5923 | Jain Scientific |
| Blue flame burner | Rocker scientific, Taiwan | dragon 200 | |
| Butanol | GLR inovations, India | GLR09.022930 | |
| C18 column | Agilent Technologies, USA | 770995-902 | |
| Centrifuge | Eppendorf, Germany | 5810R | |
| Chloroform | Merck, India | 1.94506.2521 | |
| Chloroform-d | SRL, India | 57034 | |
| Falcon tubes | Tarsons, India | 546041 | Radiation sterilized polypropylene |
| FT-IR | Thermo Fisher Scientific, USA | Nicolet iS50 | |
| Fume hood | Khera, India | 47408 | Customied |
| glacial acetic acid | Merck, India | 1.93002 | |
| Glass beads | Merck, India | 104014 | |
| Glass slides | Polar industrial Corporation, USA | Blue Star | 75 mm * 25 mm |
| Glass wool | Merk, India | 104086 | |
| Hydrochloric acid | Merck, India | 1003170510 | |
| Incubator | Thermo Scientific, USA | MaxQ600 | Shaking incubator |
| Incubator | Khera, India | Sunbim | |
| Iodine resublimed | Merck, India | 231-442-4 | resublimed Granules |
| K12 –Kruss tensiometer | Kruss Scientific, Germany | K100 | |
| Laminar air flow cabnet | Thermo Scientific, China | 1300 Series A2 | |
| LCMS | Agilent Technologies, USA | 1260 Infinity II | |
| Luria Broth | HIMEDIA, India | M575-500G | Powder |
| Methanol | Merck, India | 107018 | |
| Ninhydrin | Titan Biotech Limited, India | 1608 | |
| p- anisaldehyde | Sigma, USA | 204-602-6 | |
| Petri plate | Tarsons, India | 460090-90 MM | Radiation sterilized polypropylene |
| Saponin | Merck, India | 232-462-6 | |
| Sodium chloride | Merck, India | 231-598-3 | |
| Test tubes | Borosil, India | 9800U06 | Glass tubes |
| TLC plates | Merck, India | 1055540007 | |
| Vortex | GeNei, India | 2006114318 | |
| Water Bath | Julabo, India | SW21C |
Referências
- Desai, J. D., Banat, I. M. Microbial production of surfactants and their commercial potential. Microbiology and Molecular Biology Reviews. 61 (1), 47-64 (1997).
- Banat, I. M. Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: a review. Bioresource Technology. 51 (1), 1-12 (1995).
- Singh, A., Van Hamme, J. D., Ward, O. P. Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnology Advances. 25 (1), 99-121 (2007).
- Shah, N., Nikam, R., Gaikwad, S., Sapre, V., Kaur, J. Biosurfactant: types, detection methods, importance and applications. Indian Journal of Microbiology Research. 3 (1), 5-10 (2016).
- McClements, D. J., Gumus, C. E. Natural emulsifiers-Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Advances in Colloid and Interface Science. 234, 3-26 (2016).
- Nguyen, T. T., Youssef, N. H., McInerney, M. J., Sabatini, D. A. Rhamnolipid biosurfactant mixtures for environmental remediation. Water Research. 42 (6-7), 1735-1743 (2008).
- Maier, R. M., Soberon-Chavez, G. Pseudomonas aeruginosa rhamnolipids: biosynthesis and potential applications. Applied Microbiology and Biotechnology. 54 (5), 625-633 (2000).
- Banat, I. M., Makkar, R. S., Cameotra, S. S. Potential commercial applications of microbial surfactants. Applied Microbiology and Biotechnology. 53 (5), 495-508 (2000).
- Mulugeta, K., Kamaraj, M., Tafesse, M., Aravind, J. A review on production, properties, and applications of microbial surfactants as a promising biomolecule for environmental applications. Strategies and Tools for Pollutant Mitigation: Avenues to a Cleaner Environment. , 3-28 (2021).
- Sharma, J., Sundar, D., Srivastava, P. Biosurfactants: Potential agents for controlling cellular communication, motility, and antagonism. Frontiers in Molecular Biosciences. 8, 727070 (2021).
- Vijayakumar, S., Saravanan, V. Biosurfactants-types, sources and applications. Research Journal of Microbiology. 10 (5), 181-192 (2015).
- Curiel-Maciel, N. F., et al. Characterization of enterobacter cloacae BAGM01 producing a thermostable and alkaline-tolerant rhamnolipid biosurfactant from the Gulf of Mexico. Marine Biotechnology. 23 (1), 106-126 (2021).
- Nikolova, C., Gutierrez, T. Biosurfactants and their applications in the oil and gas industry: current state of knowledge and future perspectives. Frontiers in Bioengineering and Biotechnology. 9, (2021).
- Rastogi, S., Tiwari, S., Ratna, S., Kumar, R. Utilization of agro-industrial waste for biosurfactant production under submerged fermentation and its synergistic application in biosorption of Pb2. Bioresource Technology Reports. 15, 100706 (2021).
- Zargar, A. N., Lymperatou, A., Skiadas, I., Kumar, M., Srivastava, P. Structural and functional characterization of a novel biosurfactant from Bacillus sp. IITD106. Journal of Hazardous Materials. 423, 127201 (2022).
- Adnan, M., et al. Functional and structural characterization of pediococcus pentosaceus-derived biosurfactant and its biomedical potential against bacterial adhesion, quorum sensing, and biofilm formation. Antibiotics. 10 (11), 1371 (2021).
- Du Nouy, P. L. A new apparatus for measuring surface tension. The Journal of General Physiology. 1 (5), 521-524 (1919).
- Akbari, S., Abdurahman, N. H., Yunus, R. M., Fayaz, F., Alara, O. R. Biosurfactants-a new frontier for social and environmental safety: a mini review. Biotechnology Research and Innovation. 2 (1), 81-90 (2018).
- Bicca, F. C., Fleck, L. C., Ayub, M. A. Z. Production of biosurfactant by hydrocarbon degrading Rhodococcus ruber and Rhodococcus erythropolis. Revista de Microbiologia. 30 (3), 231-236 (1999).
- Kuyukina, M. S., et al. Recovery of Rhodococcus biosurfactants using methyl tertiary-butyl ether extraction. Journal of Microbiological Methods. 46 (2), 149-156 (2001).
- Philp, J., et al. Alkanotrophic Rhodococcus ruber as a biosurfactant producer. Applied Microbiology and Biotechnology. 59 (2), 318-324 (2002).
- Mutalik, S. R., Vaidya, B. K., Joshi, R. M., Desai, K. M., Nene, S. N. Use of response surface optimization for the production of biosurfactant from Rhodococcus spp. MTCC 2574. Bioresource Technology. 99 (16), 7875-7880 (2008).
- Shavandi, M., Mohebali, G., Haddadi, A., Shakarami, H., Nuhi, A. Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp. strain TA6. Colloids and Surfaces B, Biointerfaces. 82 (2), 477-482 (2011).
- White, D., Hird, L., Ali, S. Production and characterization of a trehalolipid biosurfactant produced by the novel marine bacterium Rhodococcus sp., strain PML026. Journal of Applied Microbiology. 115 (3), 744-755 (2013).
- Najafi, A., et al. Interactive optimization of biosurfactant production by Paenibacillus alvei ARN63 isolated from an Iranian oil well. Colloids and Surfaces. B, Biointerfaces. 82 (1), 33-39 (2011).
- Bezza, F. A., Chirwa, E. M. N. Pyrene biodegradation enhancement potential of lipopeptide biosurfactant produced by Paenibacillus dendritiformis CN5 strain. Journal of Hazardous Materials. 321, 218-227 (2017).
- Jimoh, A. A., Lin, J. Biotechnological applications of Paenibacillus sp. D9 lipopeptide biosurfactant produced in low-cost substrates. Applied Biochemistry and Biotechnology. 191 (3), 921-941 (2020).
- Liang, T. -. W., et al. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Applied Biochemistry and Biotechnology. 172 (2), 933-950 (2014).
- Mesbaiah, F. Z., et al. Preliminary characterization of biosurfactant produced by a PAH-degrading Paenibacillus sp. under thermophilic conditions. Environmental Science and Pollution Research. 23 (14), 14221-14230 (2016).
- Quinn, G. A., Maloy, A. P., McClean, S., Carney, B., Slater, J. W. Lipopeptide biosurfactants from Paenibacillus polymyxa inhibit single and mixed species biofilms. Biofouling. 28 (10), 1151-1166 (2012).
- Gudiña, E. J., et al. Novel bioemulsifier produced by a Paenibacillus strain isolated from crude oil. Microbial Cell Factories. 14 (1), 1-11 (2015).
- Pradhan, A. K., Pradhan, N., Sukla, L. B., Panda, P. K., Mishra, B. K. Inhibition of pathogenic bacterial biofilm by biosurfactant produced by Lysinibacillus fusiformis S9. Bioprocess and Biosystems Engineering. 37 (2), 139-149 (2014).
- Manchola, L., Dussán, J. Lysinibacillus sphaericus and Geobacillus sp biodegradation of petroleum hydrocarbons and biosurfactant production. Remediation Journal. 25 (1), 85-100 (2014).
- Bhardwaj, G., Cameotra, S. S., Chopra, H. K. Biosurfactant from Lysinibacillus chungkukjangi from rice bran oil sludge and potential applications. Journal of Surfactants and Detergents. 19 (5), 957-965 (2016).
- Gaur, V. K., et al. Rhamnolipid from a Lysinibacillus sphaericus strain IITR51 and its potential application for dissolution of hydrophobic pesticides. Bioresource Technology. 272, 19-25 (2019).
- Habib, S., et al. Production of lipopeptide biosurfactant by a hydrocarbon-degrading Antarctic Rhodococcus. International Journal of Molecular Sciences. 21 (17), 6138 (2020).
- Shao, P., Ma, H., Zhu, J., Qiu, Q. Impact of ionic strength on physicochemical stability of o/w emulsions stabilized by Ulva fasciata polysaccharide. Food Hydrocolloids. 69, 202-209 (2017).
- . Overview of DLVO theory Available from: https://archive-ouverte.unige.ch/unige:148595 (2014)
- Kazemzadeh, Y., Ismail, I., Rezvani, H., Sharifi, M., Riazi, M. Experimental investigation of stability of water in oil emulsions at reservoir conditions: Effect of ion type, ion concentration, and system pressure. Fuel. 243, 15-27 (2019).
- Chong, H., Li, Q. Microbial production of rhamnolipids: opportunities, challenges and strategies. Microbial Cell Factories. 16 (1), 1-12 (2017).
- Zeng, G., et al. Co-degradation with glucose of four surfactants, CTAB, Triton X-100, SDS and Rhamnolipid, in liquid culture media and compost matrix. Biodegradation. 18 (3), 303-310 (2007).
- Liu, G., et al. Advances in applications of rhamnolipids biosurfactant in environmental remediation: a review. Biotechnology and Bioengineering. 115 (4), 796-814 (2018).
- John, W. C., Ogbonna, I. O., Gberikon, G. M., Iheukwumere, C. C. Evaluation of biosurfactant production potential of Lysinibacillus fusiformis MK559526 isolated from automobile-mechanic-workshop soil. Brazilian Journal of Microbiology. 52 (2), 663-674 (2021).
- Naing, K. W., et al. Isolation and characterization of an antimicrobial lipopeptide produced by Paenibacillus ehimensis MA2012. Journal of Basic Microbiology. 55 (7), 857-868 (2015).
- Wittgens, A., et al. Novel insights into biosynthesis and uptake of rhamnolipids and their precursors. Applied Microbiology and Biotechnology. 101 (7), 2865-2878 (2017).
- Rahman, K., Rahman, T. J., McClean, S., Marchant, R., Banat, I. M. Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low-cost raw materials. Biotechnology Progress. 18 (6), 1277-1281 (2002).
- Bahia, F. M., et al. Rhamnolipids production from sucrose by engineered Saccharomyces cerevisiae. Scientific Reports. 8 (1), 1-10 (2018).
- Kim, C. H., et al. Desorption and solubilization of anthracene by a rhamnolipid biosurfactant from Rhodococcus fascians. Water Environment Research. 91 (8), 739-747 (2019).
- Nalini, S., Parthasarathi, R. Optimization of rhamnolipid biosurfactant production from Serratia rubidaea SNAU02 under solid-state fermentation and its biocontrol efficacy against Fusarium wilt of eggplant. Annals of Agrarian Science. 16 (2), 108-115 (2018).
- Wang, Q., et al. Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnology and Bioengineering. 98 (4), 842-853 (2007).
- Câmara, J., Sousa, M., Neto, E. B., Oliveira, M. Application of rhamnolipid biosurfactant produced by Pseudomonas aeruginosa in microbial-enhanced oil recovery (MEOR). Journal of Petroleum Exploration and Production Technology. 9 (3), 2333-2341 (2019).
- Amani, H., Mehrnia, M. R., Sarrafzadeh, M. H., Haghighi, M., Soudi, M. R. Scale up and application of biosurfactant from Bacillus subtilis in enhanced oil recovery. Applied Biochemistry and Biotechnology. 162 (2), 510-523 (2010).
- Gudiña, E. J., et al. Bioconversion of agro-industrial by-products in rhamnolipids toward applications in enhanced oil recovery and bioremediation. Bioresource Technology. 177, 87-93 (2015).
- Sun, G., Hu, J., Wang, Z., Li, X., Wang, W. Dynamic investigation of microbial activity in microbial enhanced oil recovery (MEOR). Petroleum Science and Technology. 36 (16), 1265-1271 (2018).
- Jha, S. S., Joshi, S. J., SJ, G. Lipopeptide production by Bacillus subtilis R1 and its possible applications. Brazilian Journal of Microbiology. 47 (4), 955-964 (2016).
- Darvishi, P., Ayatollahi, S., Mowla, D., Niazi, A. Biosurfactant production under extreme environmental conditions by an efficient microbial consortium, ERCPPI-2. Colloids and Surfaces. B, Biointerfaces. 84 (2), 292-300 (2011).
- Al-Wahaibi, Y., et al. Biosurfactant production by Bacillus subtilis B30 and its application in enhancing oil recovery. Colloids and Surfaces. B, Biointerfaces. 114, 324-333 (2014).
- Moutinho, L. F., Moura, F. R., Silvestre, R. C., Romão-Dumaresq, A. S. Microbial biosurfactants: A broad analysis of properties, applications, biosynthesis, and techno-economical assessment of rhamnolipid production. Biotechnology Progress. 37 (2), 3093 (2021).
- Youssef, N., Simpson, D. R., McInerney, M. J., Duncan, K. E. In-situ lipopeptide biosurfactant production by Bacillus strains correlates with improved oil recovery in two oil wells approaching their economic limit of production. International Biodeterioration & Biodegradation. 81, 127-132 (2013).
- Ruckenstein, E., Nagarajan, R. Critical micelle concentration and the transition point for micellar size distribution. The Journal of Physical Chemistry. 85 (20), 3010-3014 (1981).
- de Araujo, L. L., et al. Microbial enhanced oil recovery using a biosurfactant produced by Bacillus safensis isolated from mangrove microbiota-Part I biosurfactant characterization and oil displacement test. Journal of Petroleum Science and Engineering. 180, 950-957 (2019).
- Banat, I. M., De Rienzo, M. A. D., Quinn, G. A. Microbial biofilms: biosurfactants as antibiofilm agents. Applied Microbiology and Biotechnology. 98 (24), 9915-9929 (2014).
- Klosowska-Chomiczewska, I., Medrzycka, K., Karpenko, E. Biosurfactants-biodegradability, toxicity, efficiency in comparison with synthetic surfactants. Research and Application of New Technologies in Wastewater Treatment and Municipal Solid Waste Disposal in Ukraine, Sweden, and Poland. 17, 141-149 (2013).
- Fernandes, P. A. V., et al. Antimicrobial activity of surfactants produced by Bacillus subtilis R14 against multidrug-resistant bacteria. Brazilian Journal of Microbiology. 38 (4), 704-709 (2007).
- Santos, D. K. F., Rufino, R. D., Luna, J. M., Santos, V. A., Sarubbo, L. A. Biosurfactants: multifunctional biomolecules of the 21st century. International Journal of Molecular Sciences. 17 (3), 401 (2016).

