Microscopy Techniques for Interpreting Fungal Colonization in Mycoheterotrophic Plants Tissues and Symbiotic Germination of Seeds
Summary
This protocol aims to provide detailed procedures for collecting, fixing, and maintaining mycoheterotrophic plant samples, applying different microscopy techniques such as scanning and transmission electron microscopy, light, confocal, and fluorescence microscopy to study fungal colonization in plants tissues and seeds germinated with mycorrhizal fungi.
Abstract
Structural botany is an indispensable perspective to fully understand the ecology, physiology, development, and evolution of plants. When researching mycoheterotrophic plants (i.e., plants that obtain carbon from fungi), remarkable aspects of their structural adaptations, the patterns of tissue colonization by fungi, and the morphoanatomy of subterranean organs can enlighten their developmental strategies and their relationships with hyphae, the source of nutrients. Another important role of symbiotic fungi is related to the germination of orchid seeds; all Orchidaceae species are mycoheterotrophic during germination and seedling stage (initial mycoheterotrophy), even the ones that photosynthesize in adult stages. Due to the lack of nutritional reserves in orchid seeds, fungal symbionts are essential to provide substrates and enable germination. Analyzing germination stages by structural perspectives can also answer important questions regarding the fungi interaction with the seeds. Different imaging techniques can be applied to unveil fungi endophytes in plant tissues, as are proposed in this article. Freehand and thin sections of plant organs can be stained and then observed using light microscopy. A fluorochrome conjugated to wheat germ agglutinin can be applied to the fungi and co-incubated with Calcofluor White to highlight plant cell walls in confocal microscopy. In addition, the methodologies of scanning and transmission electron microscopy are detailed for mycoheterotrophic orchids, and the possibilities of applying such protocols in related plants is explored. Symbiotic germination of orchid seeds (i.e., in the presence of mycorrhizal fungi) is described in the protocol in detail, along with possibilities of preparing the structures obtained from different stages of germination for analyses with light, confocal, and electron microscopy.
Introduction
Structural research in botany, covering plant morphology and anatomy, is basic in understanding the whole organism1,2, and provides indispensable perspectives to integrate and contribute to knowledge regarding the ecology, physiology, development, and evolution of plants3. Methods in plant morphology and anatomy currently comprise protocols, equipment, and knowledge developed recently as well as more than a century ago2. The continuous execution and adaptation of classical methods (e.g., light microscopy) along with more recent techniques (e.g., confocal microscopy, X-ray microtomography) have the same essential basis: theoretical knowledge enabling the development of a methodology.
The main tool in plant anatomy and morphology is the image. Despite the misconception that such analyses are simple observations, giving space to subjective interpretations2, analyzing and understanding images in this area requires knowledge of the methods applied (the equipment, type of analysis, methodological procedures), cell components, histochemistry, and the plant body (tissue organization and function, ontogeny, morphological adaptations). Interpreting the images obtained via a variety of methods can lead to correlating form and function, deciphering the chemical composition of a structure, corroborating in describing taxa, understanding infections by phytopathogens, and other such assessments.
When researching mycoheterotrophic (MH) plants (i.e., non-photosynthetic plants that obtain carbon from mycorrhizal fungi4,5), remarkable aspects of their structural adaptations, the patterns of tissue colonization by fungi, and the morphoanatomy of subterranean organs can enlighten their development strategies and relationships with hyphae, which are the source of nutrients. The subterranean organs of MH plants usually show important adaptations related to their association with soil fungi, hence it is essential to perform these anatomical and morphological investigations6. MH species' aerial organs should not be ignored, as endophytes can be also present in these tissues, even if they are not mycorrhizal fungi (personal observations, not published yet).
Besides the well-established essentiality of mycorrhizal fungi association with MH species during their entire life cycle7, every orchid species, even the autotrophic ones, have an initial obligate mycoheterotrophic stage in natural environments. It occurs because the orchids' embryo is undifferentiated and lacks an endosperm or cotyledons, thus being incapable of developing and establishing itself in natural environments without the nutritional support of fungal partners4,8. Considering that, symbiotic germination protocols can be applied not only to MH species but also to photosynthesizing orchids, aiming to investigate orchid-fungus specificity in germination and protocorm development, a vastly applied methodology in initiatives for the conservation of threatened species9,10,11.
In this methods assembly, we describe important steps involved in collecting, fixing, and storing MH plant samples for anatomical studies (section 1), surface analysis and sample selection (section 2), sectioning methods (freehand: section 3, microtomy: section 4, cryomicrotomy: section 5), staining and mounting (section 6), fluorescence and confocal microscopy of fungal endophytes (section 7), scanning electron microscopy (section 8), and transmission electron microscopy (section 9). Additionally, we describe a symbiotic germination method for orchid seeds (MH and autotrophic, section 10), as the imaging methods previously mentioned can be successfully applied to analyze fungal colonization of seeds, protocorms, and seedlings in the germination process.
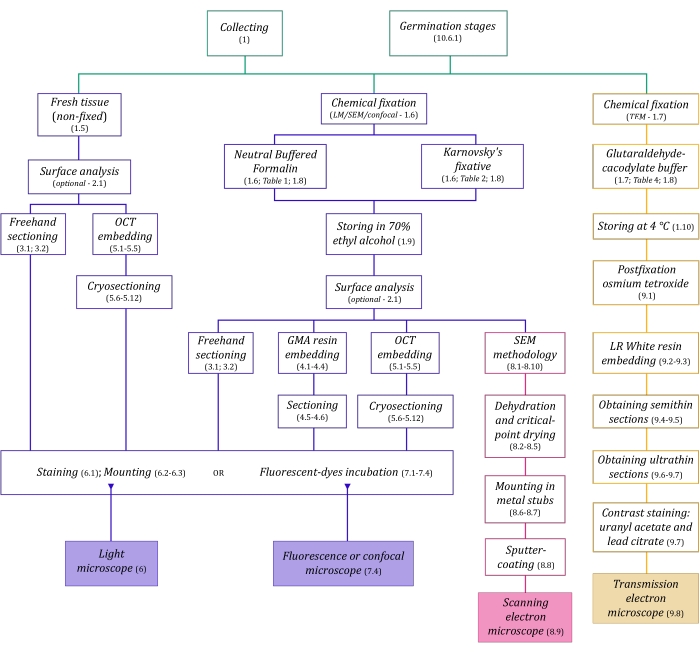
Figure 1: Schematic summarization of imaging methods. The schematics provide indications of protocol steps in which they are detailed. Abbreviations: GMA = glycol methacrylate, OCT = optimal cutting temperature compound, SEM = scanning electron microscopy. Please click here to view a larger version of this figure.
The microscopy techniques described here in detail (Figure 1) are preceded by the following essential steps: collecting, fixing, dehydrating, embedding, and sectioning samples. As the steps are variable (Figure 1) depending on the chosen technique(s), it is important to think ahead, considering the fixatives to be prepared and transported to the collection site, how the samples must be prepared before fixing, the dehydration processes to be used (section 1), and different embedding possibilities and sectioning methods (sections 4, 5, and 9). Figure 1 summarizes sequentially all the steps required for each microscopy technique thoroughly described below.
Protocol
Representative Results
Discussion
Image analyses in plant anatomy and morphology have an important potential to fulfil objectives and help understand the relationships between mycoheterotrophic plants and their indispensable fungal endophytes, as demonstrated by studies of subterranean organs6,40, structural analyses of symbiotic germination of seeds39, and aerial and reproductive structures41. Structural botany, despite having lost its prestige and…
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors thank funding from FAEPEX and FAPESP (2015/26479-6). MPP thanks Capes for his master's degree scholarship (process 88887.600591/2021-00) and CNPq. JLSM thanks CNPq for productivity grants (303664/2020-7). The authors also thank the access to equipment and assistance provided by LME (Laboratory of Electron Microscopy – IB/Unicamp), INFABiC (National Institute of Science and Technology on Photonics Applied to Cell Biology – Unicamp), and LaBiVasc (Laboratory of Vascular Biology – DBEF/IB/Unicamp); LAMEB (UFSC) and Eliana de Medeiros Oliveira (UFSC) for contributions to cryoprotection protocol; LME for contributions to TEM protocol.
Materials
| Acetone | Sigma-Aldrich | 179124 | (for SEM stubs mounting) |
| Agar-agar (AA) | Sigma-Aldrich | A1296 | (for seeds germination tests) |
| Calcofluor White Stain | Sigma-Aldrich | 18909 | fluorescent dye (detects cellulose) |
| Citrate Buffer Solution, 0.09M pH 4.8 | Sigma-Aldrich | C2488 | (for toluidine blue O staining) |
| Conductive Double-Sided Carbon Tape | Fisher Scientific | 50-285-81 | (for SEM) |
| Confocal Microscope | Zeiss | (any model) | |
| Copper Grids | Sigma-Aldrich | G4776 | (for TEM) |
| Critical-point dryer | Balzers | (any model) | |
| Cryostat | Leica Biosystems | (any model) | |
| Dissecting microscope | Leica Biosystems | (= stereomicroscope, any model) | |
| Entellan | Sigma-Aldrich | 107960 | rapid mounting medium for microscopy |
| Ethyl alcohol, pure (≥99.5%) | Sigma-Aldrich | 459836 | (= ethanol, for dehydration processes) |
| Formaldehyde solution, 37% | Sigma-Aldrich | 252549 | (for NBF solution preparation) |
| Formalin solution, neutral buffered, 10% | Sigma-Aldrich | HT501128 | histological tissue fixative |
| Gelatin capsules for TEM | Fisher Scientific | 50-248-71 | (for resin polymerisation in TEM) |
| Gelatin solution, 2% in H2O | Sigma-Aldrich | G1393 | (dilute for slides preparation – OCT adherence) |
| Glutaraldehyde solution, 25% | Sigma-Aldrich | G6257 | (for Karnovsky’s solution preparation) |
| HistoResin | Leica Biosystems | 14702231731 | glycol methacrylate (GMA) embedding kit |
| Iodine | Sigma-Aldrich | 207772 | (for Lugol solution preparation) |
| Lead(II) nitrate | Sigma-Aldrich | 228621 | Pb(NO3)2 (for TEM contrast staining) |
| Light Microscope | Olympus | (any model) | |
| LR White acrylic resin | Sigma-Aldrich | L9774 | hydrophilic acrylic resin for TEM |
| Lugol solution | Sigma-Aldrich | 62650 | (for staining) |
| Metal stubs for specimen mounts | Rave Scientific | (for SEM, different models) | |
| Microtome | Leica Biosystems | manual rotary microtome or other model | |
| Oatmeal agar (OMA) | Millipore | O3506 | (for seeds germination tests) |
| OCT Compound, Tissue-Tek | Sakura Finetek USA | 4583 | embedding medium for frozen tissues |
| Osmium tetroxide | Sigma-Aldrich | 201030 | OsO4 (for TEM postfixation) |
| Parafilm M | Sigma-Aldrich | P7793 | sealing thermoplastic film |
| Paraformaldehyde | Sigma-Aldrich | 158127 | (for Karnovsky’s solution preparation) |
| Poly-L-lysine solution, 0.1% in H2O | Sigma-Aldrich | P8920 | (for slides preparation – OCT adherence) |
| Poly-Prep Slides | Sigma-Aldrich | P0425 | poly-L-lysine coated glass slides |
| Polyethylene Molding Cup Trays | Polysciences | 17177A-3 | (6x8x5 mm, for embbeding samples in GMA resin) |
| Polyethylene Molding Cup Trays | Polysciences | 17177C-3 | (13x19x5 mm, for embbeding samples in GMA resin) |
| Potassium iodide | Sigma-Aldrich | 221945 | (for Lugol solution preparation) |
| Potato Dextrose Agar (PDA) | Millipore | 70139 | (for seeds germination tests) |
| Scanning Electron Microscope | Jeol | (any model) | |
| Silane [(3-Aminopropyl)triethoxysilane] | Sigma-Aldrich | A3648 | (for slides preparation – OCT adherence) |
| Silane-Prep Slides | Sigma-Aldrich | S4651 | glass slides coated with silane |
| Silica gel orange, granular | Supelco | 10087 | (for dessicating processes) |
| Sodium cacodylate trihydrate | Sigma-Aldrich | C0250 | (for glutaraldehyde-sodium cacodylate buffer) |
| Sodium hydroxide | Sigma-Aldrich | S5881 | NaOH (for Karnovsky’s solution preparation and TEM contrast staining) |
| Sodium hypochlorite solution | Sigma-Aldrich | 425044 | NaClO (for seeds surface disinfection) |
| Sodium phosphate dibasic, anhydrous | Sigma-Aldrich | 71640 | Na2HPO4 (for NBF solution and PB preparation) |
| Sodium phosphate monobasic monohydrate | Sigma-Aldrich | S9638 | NaH2PO4·H2O (for NBF and PB) |
| Sputter coater | Balzers | (any model) | |
| Sucrose | Sigma-Aldrich | S0389 | C12H22O11 (for cryoprotection and germination test) |
| Sudan III | Sigma-Aldrich | S4131 | (for staining) |
| Sudan IV | Sigma-Aldrich | 198102 | (for staining) |
| Sudan Black B | Sigma-Aldrich | 199664 | (for staining) |
| Syringe | (3 mL, any brand, for TEM contrast staining) | ||
| Syringe Filter Unit, Millex-GV 0.22 µm | Millipore | SLGV033R | PVDF, 33 mm, gamma sterilized (for TEM contrast staining) |
| Tek Bond Super Glue 793 | Tek Bond Saint-Gobain | 78072720018 | liquid cyanoacrylate adhesive, medium viscosity |
| Toluidine Blue O | Sigma-Aldrich | T3260 | (for staining) |
| Transmission Electron Microscope | Jeol | (any model) | |
| Triphenyltetrazolium chloride | Sigma-Aldrich | T8877 | (for the tetrazolium test in seeds germination) |
| Trisodium citrate dihydrate | Sigma-Aldrich | S1804 | Na3(C6H5O7)·2H2O (for TEM contrast staining) |
| Ultramicrotome | Leica Biosystems | (any model) | |
| Uranyl acetate | Fisher Scientific | 18-607-645 | UO2(CH3COO)2 (for TEM contrast staining) |
| Vacuum pump | (any model) | ||
| Wheat Germ Agglutinin, Alexa Fluor 488 Conjugate | TermoFisher Scientific | W11261 | fluorescent dye-conjugated lectin (detects sialic acid and N-acetylglucosaminyl residues) |
Referências
- Evert, R. F. . Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development. , (2006).
- Yeung, E. C. T., Stasolla, C., Sumner, M. J., Huang, B. Q. . Plant Microtechniques and Protocols. , (2015).
- Sokoloff, D. D., Jura-Morawiec, J., Zoric, L., Fay, M. F. Plant anatomy: at the heart of modern botany. Botanical Journal of the Linnean Society. 195 (3), 249-253 (2021).
- Leake, J. R. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytologist. 127 (2), 171-216 (1994).
- Bidartondo, M. I. The evolutionary ecology of myco-heterotrophy. New Phytologist. 167 (2), 335-352 (2005).
- Imhof, S., Massicotte, H. B., Melville, L. H., Peterson, R. L. Subterranean morphology and mycorrhizal structures. Mycoheterotrophy. , 157-214 (2013).
- Rasmussen, H. N., Rasmussen, F. N. Orchid mycorrhiza: implications of a mycophagous life style. Oikos. 118 (3), 334-345 (2009).
- Rasmussen, H. N., Dixon, K. W., Jersáková, J., Těšitelová, T. Germination and seedling establishment in orchids: a complex of requirements. Annals of Botany. 116 (3), 391-402 (2015).
- Zettler, L. W. Terrestrial orchid conservation by symbiotic seed germination: techniques and perspectives. Selbyana. 18 (2), 188-194 (1997).
- Stewart, S. L., Kane, M. E. Symbiotic seed germination and evidence for in vitro mycobiont specificity in Spiranthes brevilabris (Orchidaceae) and its implications for species-level conservation. In Vitro Cellular & Developmental Biology – Plant. 43 (3), 178-186 (2007).
- Zhao, D. -. K., et al. Orchid reintroduction based on seed germination-promoting mycorrhizal fungi derived from protocorms or seedlings. Frontiers in Plant Science. 12, 701152 (2021).
- Selosse, M. A., Roy, M. Green plants that feed on fungi: facts and questions about mixotrophy. Trends in Plant Science. 14 (2), 64-70 (2009).
- Merckx, V. S. F. T., Mennes, C. B., Peay, K. G., Geml, J. Evolution and diversification. Mycoheterotrophy: The Biology of Plants Living on Fungi. , 215-244 (2013).
- Boon, M. E., Drijver, J. Routine Cytological Staining Techniques: Theoretical Background and Practice. Macmillan International Higher Education. , (1986).
- Karnovsky, M. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. Journal of Cell Biology. 27 (2), 137-138 (1964).
- Hayat, M. . Fixation for Electron Microscopy. , (1981).
- Roland, J. C., Vian, B. General preparation and staining of thin sections. Electron Microscopy of Plant Cells. 1, 675 (1991).
- Gerrits, P. O., Horobin, R. W. Glycol methacrylate embedding for light microscopy: basic principles and trouble-shooting. Journal of Histotechnology. 19 (4), 297-311 (1996).
- Zhang, Z., Niu, L., Chen, X., Xu, X., Ru, Z. Improvement of plant cryosection. Frontiers in Biology. 7 (4), 374-377 (2012).
- BeneŠ, K. On the media improving freeze-sectioning of plant material. Biologia Plantarum. 15 (1), 50-56 (1973).
- Fischer, A. H., Jacobson, K. A., Rose, J., Zeller, R. Preparation of slides and coverslips for microscopy. Cold Spring Harbor Protocols. 2008 (5), (2008).
- Sakai, W. S. Simple method for differential staining of paraffin embedded plant material using toluidine blue O. Stain Technology. 48 (5), 247-249 (1973).
- O’Brien, T., Feder, N., McCully, M. E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma. 59 (2), 368-373 (1964).
- Ventrella, M. C., Almeida, A. L., Nery, L. A., Coelho, V. P. d. e. M. Métodos Histoquímicos Aplicados às Sementes. Universidade Federal de Viçosa. , (2013).
- Pearse, A. G. E. . Histochemistry, Theoretical and Applied. , (1960).
- Andrade-Linares, D. R., Franken, P. Fungal endophytes in plant roots: taxonomy, colonization patterns, and functions. Symbiotic Endophytes. , 311-334 (2013).
- Wymer, C. L., Beven, A. F., Boudonck, K., Lloyd, C. W. Confocal microscopy of plant cells. Confocal Microscopy Methods and Protocols. , 103-130 (1999).
- Marques, J. P. R., Soares, M. K. M. Manual de Técnicas Aplicadas à Histopatologia Vegetal. FEALQ. , (2021).
- Navarro, B. L., Marques, J. P. R., Appezzato-da-Glória, B., Spósito, M. B. Histopathology of Phakopsora euvitis on Vitis vinifera. European Journal of Plant Pathology. 154 (4), 1185-1193 (2019).
- Marques, J. P. R., et al. Sugarcane cell wall-associated defense responses to infection by Sporisorium scitamineum. Frontiers in Plant Science. 9, 698 (2018).
- Jeffree, C. E., Read, N. D. Ambient-and low-temperature scanning electron microscopy. Electron Microscopy of Plant Cells. , 313-413 (1991).
- Bozzola, J. J., Russell, L. D. . Electron Microscopy: Principles and Techniques for Biologists. , (1999).
- Murray, S. Basic transmission and scanning electron microscopy. Introduction to electron Microscopy for Biologists. , 3-18 (2008).
- . Glossary of TEM terms Available from: https://www.jeol.co.jp/en/words/emterms/ (2021)
- Seaton, P. T., et al. Orchid seed and pollen: a toolkit for long-term storage, viability assessment and conservation. Orchid Propagation: From Laboratories to Greenhouses—Methods and Protocols. , 71-98 (2018).
- Otero, J. T., Ackerman, J. D., Bayman, P. Differences in mycorrhizal preferences between two tropical orchids. Molecular Ecology. 13 (8), 2393-2404 (2004).
- Koch, R. A., et al. Marasmioid rhizomorphs in bird nests: Species diversity, functional specificity, and new species from the tropics. Mycologia. 112 (6), 1086-1103 (2020).
- Webster, J., Weber, R. . Introduction to Fungi. , (2007).
- Sisti, L. S., et al. The role of non-mycorrhizal fungi in germination of the mycoheterotrophic orchid Pogoniopsis schenckii Cogn. Frontiers in Plant Science. 10, 1589 (2019).
- Martos, F., et al. Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytologist. 184 (3), 668-681 (2009).
- Alves, M. F., et al. Reproductive development and genetic structure of the mycoheterotrophic orchid Pogoniopsis schenckii Cogn. BMC Plant Biology. 21 (1), 332 (2021).
- Merckx, V. S. F. T. Mycoheterotrophy: an introduction. Mycoheterotrophy: The Biology of Plants Living on Fungi. , 1-17 (2013).
- Hall, J. L., Hawes, C. . Electron Microscopy of Plant Cells. , (1991).

