Efficient Generation of Murine Chimeric Antigen Receptor (CAR)-T Cells
Summary
This protocol streamlines retroviral vector production and murine T cell transduction, facilitating the efficient generation of mouse CAR-T cells.
Abstract
Engineered cell therapies utilizing chimeric antigen receptor (CAR)-T cells have achieved remarkable effectiveness in individuals with hematological malignancies and are presently undergoing development for the treatment of diverse solid tumors. So far, the preliminary evaluation of novel CAR-T cell products has predominantly taken place in xenograft tumor models using immunodeficient mice. This approach is chosen to facilitate the successful engraftment of human CAR-T cells in the experimental setting. However, syngeneic mouse models, in which tumors and CAR-T cells are derived from the same mouse strain, allow evaluation of new CAR technologies in the context of a functional immune system and comprehensive tumor microenvironment (TME). The protocol described here aims to streamline the process of mouse CAR-T cell generation by presenting standardized methods for retroviral transduction and ex vivo T cell culture. The methods described in this protocol can be applied to other CAR constructs beyond the ones used in this study to enable routine evaluation of new CAR technologies in immune-competent systems.
Introduction
Adoptive T cell therapies expressing chimeric antigen receptors (CARs) have revolutionized the field of cancer immunotherapy by harnessing the power of the adaptive immune system to specifically target and eliminate antigen-positive cancer cells1. While the success of CAR-T cell therapies targeting B cell malignancies has been clinically validated, preclinical studies performed in animal models remain vital for the development of new CARs targeting solid tumors. However, limited clinical efficacy has been demonstrated in solid tumor indications thus far, and it is becoming increasingly apparent that individual preclinical models do not accurately predict the pharmacodynamics and clinical efficacy of a living medicine2,3. Therefore, investigators have begun to expand the preclinical study of CAR-T cell products to include parallel assessments in xenograft and syngeneic models of human and murine cancers, respectively.
Unlike xenograft models, where human tumors and T cells are engrafted into immunodeficient mice, syngeneic models enable the examination of CAR-T cell responses in the context of a functional immune system. Specifically, immune-competent mice bearing syngeneic tumors provide a system to study the interaction between adoptively transferred T cells and context-specific milieus – including tumor-associated macrophages (TAMs) and regulatory T cells (Tregs) known to suppress T cell function in the tumor microenvironment (TME)4,5,6. Moreover, syngeneic models offer an additional platform to assess on-target, off-tumor toxicity, and CAR-T cell interaction with host factors that may lead to additional toxicities, including cytokine release syndrome7.
Despite these advantages, the number of syngeneic CAR-T cell studies remains limited. Notably, syngeneic models require autologous engineering of CAR-T cells from the same mouse strain and thus present an additional challenge due to the lack of methodology for efficient murine T cell transduction and ex vivo expansion2,8. This protocol outlines the methods to achieve stable CAR expression through the production of retroviral vectors and optimized T cell transduction. A schematic of the entire process is shown in Figure 1. The use of this approach demonstrates efficient retroviral transduction of murine CAR-T cells and the achievement of high CAR expression without the need for viral concentration through ultracentrifugation. Strategies to change the antigen-specificity of the CAR construct are discussed in addition to the co-expression of additional transgenes.
Protocol
All animal procedures were performed with approval from the Institutional Animal Care and Use Committee (Columbia University, protocols AC-AABQ5551 and AC-AAAZ4470) using 6-8-week-old female BALB/c or CF57BL/6 mice weighing between 20-25 g. The animals were obtained from a commercial source (see Table of Materials). This protocol is structured around the 'days post-activation' of murine T cells, and viral production begins on Day -2. Retrovirus can be stored at -80 °C following initial production, and for future use of this protocol, one may commence with step 2, T cell isolation and activation on Day 0.
1. Retroviral vector production
NOTE: The viral products have been made replication-defective by separation of the packaging genes into two separate plasmids (see Table of Materials), greatly reducing the likelihood of recombination events and inadvertent production of replication-competent virus.
- Prepare Phoenix Eco cells one day prior to transfection (Day -2 procedure).
- Plate approximately 1 x 107 cells in a 15 cm TC-treated plate or T150 culture flask, using 30 mL of culture medium (Phoenix Eco culture medium, as detailed in Table 1).
- Incubate overnight at 37 °C. After 18-24 h, the cells should be approximately 70% confluent and uniformly distributed to ensure a high viral yield without overgrowth.
NOTE: For optimal results, use cells with a low passage and passage them the day before plating for viral production. Do not allow Phoenix Eco cells to overgrow during routine culture.
- Prepare the transfection mix containing the lipofection and enhancer reagents, pCL-Eco (Gag/Pol), and pMSCV expression plasmid (pMSCV_PGK_mGFP28z) in reduced-serum media (see Table of Materials) (Day -1 procedure).
NOTE: Co-transfection should be performed at a 1:1 ratio of pMSCV and pCL-Eco.- Prepare Tube A: Dilute 105 µL of the transfection reagent in 3.75 mL of reduced serum medium per 15 cm plate and mix thoroughly by vortexing or pipetting up and down.
- Prepare Tube B: Dilute pMSCV expression plasmid (21 µg) and pCL-Eco (21 µg) with 90 µL of enhancer reagent into 3.75 mL of reduced serum medium per 15 cm plate. Mix well by pipetting up and down.
NOTE: If generating multiple viral products, set up a master mix containing pCL-Eco and the enhancer reagent. - Add Tube A to Tube B and mix thoroughly by pipetting. Incubate for 10-20 min at room temperature, resulting in a total volume of approximately 7.5 mL.
- Carefully remove 10 mL of cell culture media from the Phoenix Eco plate(s) and add the entire volume of the transfection mix to the remaining media by tilting the plate and pipetting dropwise. Return cells to a 37 °C incubator.
- Change the media on transfected Phoenix Eco cells 16-20 h post-transfection (Day 0 procedure).
- Pre-warm serum and β-mercaptoethanol (2-ME, see Table of Materials)-free murine T cell medium (mTCM-viral harvest, as listed in Table 1) to 37 °C using a water or bead bath.
- Carefully remove the Phoenix Eco culture medium by tilting the plate and placing the pipette tip at the bottom corner, using a vacuum if possible. Avoid over-drying the cells.
- Gently add the warmed mTCM-viral harvest medium to the side of the tilted plate by pipetting 30 mL on the slowest setting. Return cells to a 37 °C incubator.
NOTE: This step can cause Phoenix Eco cells to detach from the plate and reduce viral titers. Pre-warmed media and gentle pipetting will minimize disruption.
- Harvest the viral supernatant 48 h post-transfection (Day 1 procedure).
- Harvest the viral supernatant and filter it through 0.45 µm PVDF filters (see Table of Materials) to remove cells and debris. Aliquot and freeze the virus at -80 °C or proceed directly to Day 1 of step 3 if using fresh virus.
- Determine the viral titer (transducing units/mL) by flow cytometry, as previously described9. This step is optional.
- Determine the viral titer by small-scale transduction of 1 x 105 activated murine T cells in non-tissue culture (TC)-treated 96-well plates, pre-coated with retronectin (see Table of Materials) and loaded with threefold serial dilutions of retroviral supernatant in a final volume of 100 µL of mTCM-viral harvest medium.
NOTE: See step 2 and step 3 below for detailed instructions on T cell isolation, activation, and transduction. - Determine CAR surface expression 4-5 days post-transduction by flow cytometry.
- Calculate the viral titer according to the formula10: (N x F x D)/V, where N is the number of cells transduced, F is the frequency of CAR-positive cells, D is the dilution factor, and V is the transduction volume in mL to obtain transducing units (TU)/mL.
NOTE: Viral titer may decrease upon freeze/thaw; therefore, viral titer is ideally determined on frozen and subsequently thawed virus.
- Determine the viral titer by small-scale transduction of 1 x 105 activated murine T cells in non-tissue culture (TC)-treated 96-well plates, pre-coated with retronectin (see Table of Materials) and loaded with threefold serial dilutions of retroviral supernatant in a final volume of 100 µL of mTCM-viral harvest medium.
2. Murine T cell isolation
- Isolate and activate murine T cells (Day 0 procedure).
NOTE: These steps can be performed at room temperature (RT) or 4 °C and should be carried out in a sterile environment.- Harvest the spleen(s) from the mouse strain of interest (e.g., Balb/c, C57BL/6) as previously described11 and obtain a single-cell suspension through mechanical dissociation.
- Using the back of a sterile syringe, crush the spleen(s) through a 70-100 µm cell strainer into a 50 mL conical tube.
- After setting the syringe aside, wash the strainer with 5 mL of T cell isolation buffer (phosphate-buffered saline, PBS, supplemented with 2% fetal bovine serum, FBS).
- Repeat the mashing step and wash the strainer once more with 5 mL of T cell isolation buffer. Bring the final volume to 50 mL with T cell isolation buffer.
- Count live cells using a manual hemocytometer or an automatic cell counter by diluting at a 1:20 ratio and then mixing at 1:1 with trypan blue.
- Pellet the cells by centrifugation for 10 min at 450 x g, at 4 °C (or RT), and re-suspend spleenocytes at 1 x 108/mL if using the recommended T cell isolation kit (see Table of Materials).
NOTE: Spleenocytes can be re-strained through a 40-70 µm strainer at this stage to remove clumps.
- Isolate CD3+ T cells by negative selection following the manufacturer's instructions (see Table of Materials). After magnetic separation, transfer the isolate to a 15 mL conical tube and perform a final live cell count.
- Pellet the isolated T cells by centrifugation for 10 min at 450 x g, at 4 °C (or RT), and re-suspend them at 1 x 106/mL in mTCM-activation medium (Table 1).
- Activate T cells by adding murine anti-CD3/anti-CD28 monoclonal antibody-coated magnetic beads (see Table of Materials) at a ratio of 25 µL/1 x 106 T cells and 100 U/mL IL-2.
- Place the cells in an incubator at 37 °C and leave them overnight.
NOTE: Due to the small size of T cells, a more accurate live T cell count may be achieved by diluting at a 1:10-1:20 ratio and mixing at 1:1 with trypan blue for manual counting using a hemocytometer. Purity may be determined by flow cytometry by staining cells with a fluorescently conjugated αCD3 antibody. Each spleen will yield between 7-10 x 106 T cells depending on the age and strain of the mouse.
3. Murine T cell transduction
- Prepare plates for transduction (Day 0 procedure).
- Pre-coat non-treated sterile 24-well plates with 0.5 mL of human fibronectin transduction enhancer reagent (see Table of Materials) at a final concentration of 20-40 µg/mL by diluting it in sterile PBS and store at 4 °C overnight.
NOTE: This step may also be performed on Day 1 by coating non-treated 24-well plates with the transduction reagent and incubating them at room temperature for 2 h.
- Pre-coat non-treated sterile 24-well plates with 0.5 mL of human fibronectin transduction enhancer reagent (see Table of Materials) at a final concentration of 20-40 µg/mL by diluting it in sterile PBS and store at 4 °C overnight.
- Perform T cell transduction (Day 1 procedure).
- Prepare the pre-coated plate for transduction. Remove the transduction reagent from each well of the pre-coated 24-well plate and block with an equivalent volume of sterile-filtered PBS + 2% bovine serum albumin (BSA) (0.5 mL) for 30 min at RT.
- Wash once with 0.5-1 mL of PBS.
- Add 0.5-1 mL of neat retrovirus from step 1 or diluted based on viral titer to each pre-coated well and centrifuge for 90 min at 2,000 x g and 32 °C.
- Add 1 mL of activated T cells to each virally loaded well and centrifuge for 10 min at 450 x g and 32 °C. Return cells to a 37 °C incubator overnight.
- 24 h post-transduction, remove 1-1.5 mL of cell culture media and replace it with 1-1.5 mL of mTCM-complete and 10 ng/mL of recombinant human IL-7 and IL-15 (see Table of Materials). Return cells to a 37 °C incubator (Day 2 procedure).
NOTE: During ex vivo culture, 10 ng/mL of cytokines must be added to the culture every 48 h, and T cells should not be diluted beyond 1 x 106/mL. - 48 h post-transduction, transfer cells from the transduction plate into a fresh 24-well or 6-well plate and return cells to a 37 °C incubator (Day 3 procedure).
NOTE: T cell viability may improve by transferring cells on 24 h to 2 days post-transduction, depending on starting T cell viability and viral titer. - De-bead the T cells and confirm CAR expression (Day 5-6 procedure).
- Thoroughly re-suspend cells to dissociate activated T cells from the αCD3/CD28-coated beads (see Table of Materials) and place the cell suspension on a magnet for 30 s. Transfer the cell suspension to the desired ex vivo culture vessel and return it to a 37 °C incubator.
NOTE: T cells may be cultured in culture flasks or deep well culture plates. It is recommended to plate a minimum of 5 x 106 T cells in 30 mL of mTCM-complete medium per well in a deep well of a 6-well format plate (see Table of Materials). - Determine CAR expression by flow cytometry8.
NOTE: GFP-CAR expression was determined by incubation with 100 ng/mL of purified GFP. Incorporation of an N-terminal epitope tag will facilitate the detection of alternative CAR constructs.
- Thoroughly re-suspend cells to dissociate activated T cells from the αCD3/CD28-coated beads (see Table of Materials) and place the cell suspension on a magnet for 30 s. Transfer the cell suspension to the desired ex vivo culture vessel and return it to a 37 °C incubator.
- Perform ex vivo culture of CAR-T cells (Day 7-10 procedure).
- During ex vivo culture, maintain the cells by removing 50% of the culture medium and replacing it with fresh mTCM-complete + 2x 10 ng/mL of IL-7 and IL-15 every 48 h.
NOTE: Murine CAR-T cells are ready for use in downstream applications 7 days post-activation and should not be cryopreserved due to poor viability post-thaw.
- During ex vivo culture, maintain the cells by removing 50% of the culture medium and replacing it with fresh mTCM-complete + 2x 10 ng/mL of IL-7 and IL-15 every 48 h.
Representative Results
The protocol described here aims to standardize the process of murine T cell transduction for the generation of mouse CAR-T cells. Figure 1 provides a detailed description of the steps involved. The process begins with the production of retroviral vectors via co-transfection of viral components into Phoenix Eco cells. Figure 2 provides an image of the optimal density of Phoenix Eco cells on the day of transfection. Isolated T cells are then activated 24 h post-transfection, or 'Day 0' of this protocol, in preparation for transduction on Day 1. Following transduction, CAR-T cells are cultured in the presence of recombinant human IL-7 and IL-15 to achieve sufficient fold expansion while preserving stem cell memory populations.
The use of the recommended murine T cell isolation kit (see Table of Materials) with this protocol yields T cell purities of <98% prior to transduction, as determined by flow cytometry (Figure 3A). Moreover, reproducible CAR expression rates of 65%-75% can be achieved using this protocol and the pMSCV retroviral vector modified to express the CAR construct from a PGK promoter (Figure 3B,C). Determination of retroviral titer reveals titers of up to ~2 x 107 TU/mL and CAR expression of 74% with 1/3rd of the viral supernatant harvested following co-transfection of pMSCV with pCL-Eco (Supplementary Figure 1).
Finally, ex vivo culture with 10 ng/mL of IL-7 and IL-15 leads to approximately 15-fold expansion, resulting in ~150 x 106 T cells by Day 10 post-activation from a single spleen (Figure 3D). Culture with IL-7 and IL-15 leads to comparable CD8+ and CD4+ T cell frequencies (Figure 3E) and preserved stem cell memory populations determined by CD62L+CD44– expression following 10 days of ex vivo culture (Figure 3F).
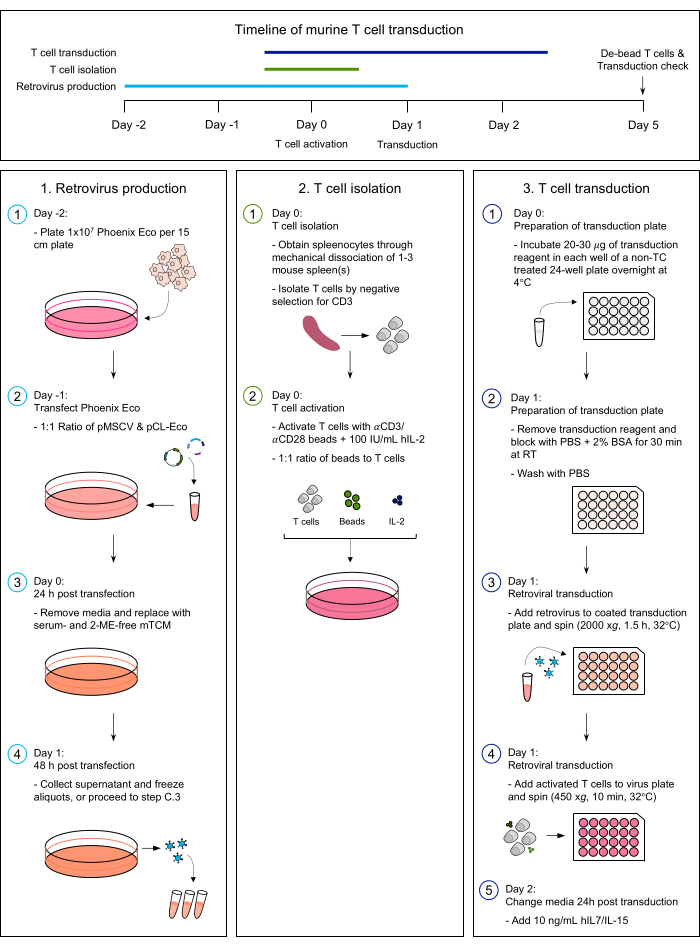
Figure 1: Protocol overview. Top panel: A schematic overview of the protocol timeline. Bottompanel: Step-by-step instructions for retroviral vector production and mouse T cell transduction. Please click here to view a larger version of this figure.
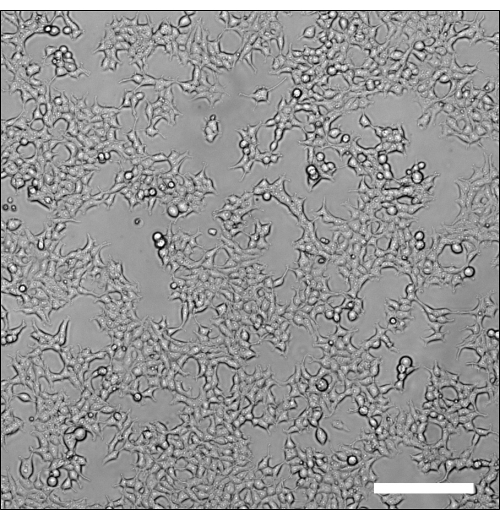
Figure 2: Optimal cell density for transfection. Brightfield microscopy image of Phoenix Eco cells at the optimal density before transfection with pMSCV and pCL-Eco. Captured using a 10x objective lens. Scale bar = 200 µm. Please click here to view a larger version of this figure.
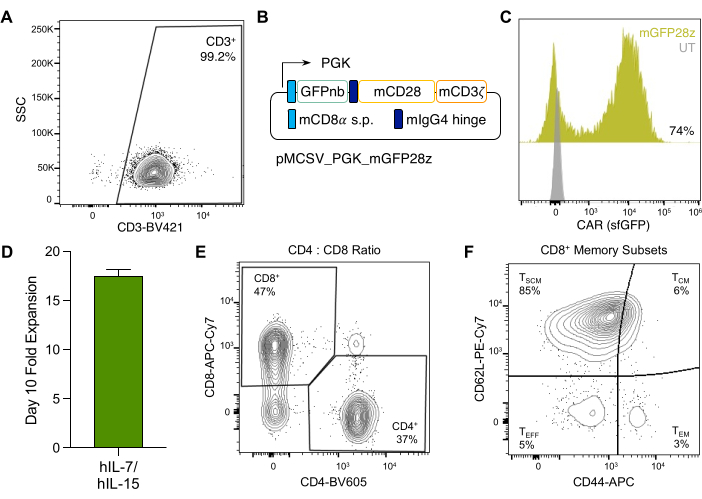
Figure 3: Murine CAR-T cell production and ex vivo culture. (A) Flow cytometry plot showing the frequency of BALB/c CD3+ cells post-murine T cell isolation. (B) Schematic representation of the pMSCV retroviral vector used for GFP-CAR expression in murine T cells. (C) Flow cytometry histograms demonstrating CAR surface expression on mouse T cells after incubation with purified sfGFP. UT, untransduced. (D) Fold expansion of CAR-T cells by day 10 post-activation and culture with recombinant human IL-17 and IL-15. Approximately 8 x 106 murine T cells were activated before transduction and reached approximately 1.28 x 108 by day 10 post-activation. (E) Flow cytometry plot showing the frequency of CD8+ and CD4+ CAR-T cells on day 10 post-activation. (F) Flow cytometry plot demonstrating the frequency of CD8+ T cell memory populations determined by CD62L and CD44 surface expression. Stem cell memory (TSCM) is characterized by CD62L+CD44–, central memory (TCM) by CD62L+CD44+, and effector memory (TEM) by CD62L–CD44+. Please click here to view a larger version of this figure.
Table 1: Cell culture media formulation. The formulation details for cell culture media used in the protocol. Please click here to download this Table.
Supplementary Figure 1: CAR surface expression and retroviral titers. (A) Flow cytometry histograms showing CAR surface expression on BALB/c T cells after incubation with purified sfGFP. Retrovirus was generated by transfecting Phoenix Eco cells with pMSCV_PGK_mGFP28z in combination with pCL-Eco and pMD2.G (I), pCL-Eco (II), or alone (III). T cells were transduced with diluted retrovirus as indicated. (B) Retroviral titers determined by CAR surface expression of transduced T cells. Transducing units (TU)/mL were calculated using the formula: (N∙F∙D)/V, where N is the number of cells transduced (1 x 105), F is the frequency of CAR+ cells, D is the dilution factor, and V is the transduction volume in mL (0.2 mL). Please click here to download this File.
Supplementary File 1: pMSCV expression vector sequence. The sequence of the GFP28z CAR and flanking sequences (italics) in the pMSCV expression vector, with NotI and BamHI enzyme sites highlighted in blue. Please click here to download this File.
Discussion
This protocol describes the steps and reagents necessary for the retroviral transduction of murine T cells to generate CAR-T cells for in vivo studies. Optimizing retroviral transduction conditions achieves robust CAR expression without the need for viral concentration through ultracentrifugation or additional reagents. However, there are multiple modifications that can be applied to this methodology.
While this protocol describes the example generation of a GFP-specific CAR, these methods can be adapted to generate mouse CAR-T cells targeting any extracellular antigen of interest. The pMSCV_PGK retroviral vector used in this study allows for the efficient release of the GFP-specific nanobody sequence by enzymatic digestion with NotI and BamHI and can be replaced with a user-defined antigen recognition domain such as an scFv, nanobody, or ligand-binding domain (Supplementary File 1). To facilitate the assessment of alternate CAR expression, it is recommended to design CARs with an N-terminal epitope tag, such as Myc (EQKLISEEDL) or Flag (DYKDDDDK) tags, which can be detected using fluorescently conjugated antibodies12. Moreover, the current study demonstrates the production of a single transgene; however, the incorporation of self-cleaving peptides (2A sequences) or internal ribosome entry sites (IRES) would enable the co-production of multiple transgenes, including additional technologies to enhance T cell function in vivo8,13,14.
Although this protocol was developed to eliminate the need for specialized equipment by omitting ultracentrifugation (24,000 x g, 2 h, 4 °C), it is worth noting that concentration of the viral supernatant can achieve higher viral titers and reduce the volume of virus required to attain high CAR expression8. This protocol additionally streamlines retroviral vector production by achieving reproducibly high viral titers (~2 x 107 TU/mL) and steady CAR expression without the need for viral titer determination prior to use. However, in addition to facilitating standardization, determination of viral titer prior to T cell transduction may also increase T cell viability by reducing potential toxicity caused by unnecessarily high viral concentrations10,15.
Supplementation with IL-7 and IL-15 is recommended to promote T cell expansion and preserve T cell memory populations; however, recombinant human IL-2 may be used as an alternative to decrease the number of necessary reagents for ex vivo culture16,17,18,19. Nonetheless, the methods for CAR-T cell generation outlined here remain limited by the relatively poor expansion of murine T cells. However, the measures to improve T cell viability discussed above could be further enhanced by including additional supplements and 2-ME in the mTCM culture medium throughout the transduction process20. While these modifications may improve T cell expansion from 15-fold to up to 20-fold20, they may result in lower transduction efficiency and reduced CAR expression.
Ultimately, the production of murine CAR-T cells enables the development of syngeneic models to evaluate CAR-T cell function within an intact immune system – facilitating a context-specific assessment of their potency, durability, and safety. Whereas models in immunodeficient mice allow for important preclinical studies of a human T cell product targeting a human tumor antigen. The combination of these complementary model systems will be indispensable for understanding the complexity of CAR-T cell biology, optimizing new CAR designs, and ultimately translating preclinical findings into effective clinical therapies.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank L. Brockmann for critical review of the manuscript. This work was supported by NIH 1R01EB030352 and UL1 TR001873.
Materials
| 0.45 μm filters | MilliporeSigma | SLHVR33RS | |
| 1 mL syringe | Fisher Scientific | 14-955-450 | |
| 1.5 mL microcentrifuge tubes | Fisher Scientific | 05-408-135 | |
| 10 mL syringe | BD | 14-823-16E | |
| 100 μm strainer | Corning | 07-201-432 | |
| 15 cm TC treated cell culture dishes | ThermoFisher Scientific | 130183 | |
| 15 mL conical tubes | Falcon | 14-959-70C | |
| 40 μm strainer | Corning | 07-201-430 | |
| 50 mL conical tubes | Falcon | 14-959-49A | |
| 70 μm strainer | Corning | 07-201-431 | |
| Attune NxT Flow Cytometer | ThermoFisher Scientific | ||
| BALB/C, 6-8 week old | Jackson Laboratory | 651 | |
| B-Mercaptoethanol | Gibco | 21985023 | |
| Bovine Serum Albumin | GOLDBIO | A-420-500 | |
| DMEM Medium | Gibco | 11965092 | |
| Dulbecco's Phosphate Buffered Saline (PBS), without Calcium and Magnesium | Gibco | 14-190-250 | |
| DynaMag-2 Magnet | Invitrogen | 12-321-D | |
| EasySep Magnet | Stemcell Technologies | 18000 | |
| EasySep Mouse T cell Isolation Kit | Stemcell Technologies | 19851 | |
| FACS buffer | BD | BDB554657 | |
| Fetal bovine serum (FBS) | Corning | MT35011CV | |
| GlutaMAX | Gibco | 35-050-061 | |
| G-Rex6 | Wilson Wolf | 80240M | |
| HEPES Buffer Solution | Gibco | 15-630-080 | |
| Human recombinant IL-15 | Miltenyi Biotec | 130-095-765 | |
| Human recombinant IL-2 | Miltenyi Biotec | 130-097-748 | |
| Human recombinant IL-7 | Miltenyi Biotec | 130-095-363 | |
| Lipofectamine 3000 | Invitrogen | L3000008 | |
| MEM Non-Essential Amino Acids Solution | Gibco | 11140-050 | |
| Mouse Anti-CD3 BV421 | Biolegend | 100228 | |
| Mouse Anti-CD3/CD28 Dynabeads | Gibco | 11-453-D | |
| Mouse Anti-CD4 BV605 | BD | 563151 | |
| Mouse Anti-CD44 APC | Biolegend | 103011 | |
| Mouse Anti-CD62L PE-Cy7 | Tonbo | SKU 60-0621-U025 | |
| Mouse Anti-CD8 APC-Cy7 | Tonbo | SKU 25-0081-U025 | |
| Nikon Ti2 with Prime 95B camera | Nikon | ||
| Non-treated 24 well plates | CytoOne | CC7672-7524 | |
| Opti-MEM | Gibco | 31-985-062 | |
| pCL-Eco | Addgene | #12371 | |
| Penicillin/Streptomycin Solution | Gibco | 15-070-063 | |
| Phoenix Eco cells | ATCC | CRL-3214 | |
| pMDG.2 | Addgene | #12259 | |
| pMSCV_PGK_GFP28z | N/A | Produced by R.LV. | |
| Purified sfGFP | N/A | Produced by R.LV. | |
| RetroNectin ('transduction reagent') | Takara Bio | T100B | |
| RPMI 1640 | Gibco | 21875 | |
| Serological pipette 10 mL | Fisher Scientific | 13-678-11E | |
| Serological pipette 25 mL | Fisher Scientific | 13-678-11 | |
| Serological pipette 5 mL | Fisher Scientific | 13-678-11D | |
| Sodium Pyruvate | Gibco | 11-360-070 | |
| TC-treated 24 well plates | Corning | 08-772-1 | |
| Trypan blue | Gibco | 15-250-061 |
Referências
- June, C. H., Sadelain, M. Chimeric antigen receptor therapy. N Engl J Med. 379 (1), 64-73 (2018).
- Duncan, B. B., Dunbar, C. E., Ishii, K. Applying a clinical lens to animal models of car-t cell therapies. Mol Ther Methods Clin Dev. 27, 17-31 (2022).
- Hou, A. J., Chen, L. C., Chen, Y. Y. Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov. 20 (7), 531-550 (2021).
- Campesato, L. F., et al. Blockade of the ahr restricts a treg-macrophage suppressive axis induced by l-kynurenine. Nat Commun. 11 (1), 4011 (2020).
- Kaneda, M. M., et al. Pi3kgamma is a molecular switch that controls immune suppression. Nature. 539 (7629), 437-442 (2016).
- Hyrenius-Wittsten, A., Roybal, K. T. Paving new roads for cars. Trends Cancer. 5 (10), 583-592 (2019).
- Giavridis, T., et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by il-1 blockade. Nat Med. 24 (6), 731-738 (2018).
- Lanitis, E., et al. Optimized gene engineering of murine CAR-T cells reveals the beneficial effects of il-15 coexpression. J Exp Med. 218 (2), e20192203 (2021).
- Lambeth, C. R., White, L. J., Johnston, R. E., De Silva, A. M. Flow cytometry-based assay for titrating dengue virus. J Clin Microbiol. 43 (7), 3267-3272 (2005).
- Agarwal, S., Wellhausen, N., Levine, B. L., June, C. H. Production of human crispr-engineered CAR-T cells. J Vis Exp. 169, e62299 (2021).
- JoVE Science Education Database. Lab Animal Research. Sterile Tissue Harvest. , (2023).
- Giordano-Attianese, G., et al. A computationally designed chimeric antigen receptor provides a small-molecule safety switch for t-cell therapy. Nat Biotechnol. 38 (4), 426-432 (2020).
- Kuhn, N. F., et al. Cd40 ligand-modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell. 35 (3), 473-488.e6 (2019).
- Jin, C., Ma, J., Ramachandran, M., Yu, D., Essand, M. CAR T cells expressing a bacterial virulence factor trigger potent bystander antitumour responses in solid cancers. Nat Biomed Eng. 6 (7), 830-841 (2022).
- Kurachi, M., et al. Optimized retroviral transduction of mouse T cells for in vivo assessment of gene function. Nat Protoc. 12 (9), 1980-1998 (2017).
- Jafarzadeh, L., Masoumi, E., Fallah-Mehrjardi, K., Mirzaei, H. R., Hadjati, J. Prolonged persistence of chimeric antigen receptor (CAR) T cell in adoptive cancer immunotherapy: Challenges and ways forward. Front Immunol. 11, 702 (2020).
- Elkassar, N., Gress, R. E. An overview of IL-7 biology and its use in immunotherapy. J Immunotoxicol. 7 (1), 1-7 (2010).
- Osinalde, N., et al. Simultaneous dissection and comparison of IL-2 and IL-15 signaling pathways by global quantitative phosphoproteomics. Proteomics. 15 (2-3), 520-531 (2015).
- Eremenko, E., et al. An optimized protocol for the retroviral transduction of mouse CD4 T cells. STAR Protoc. 2 (3), 100719 (2021).
- Lewis, M. D., et al. A reproducible method for the expansion of mouse CD8+ T lymphocytes. J Immunol Methods. 417, 134-138 (2015).

