Single-Molecule Measurement of Protein Interaction Dynamics Within Biomolecular Condensates
Summary
Many intrinsically disordered proteins have been shown to participate in the formation of highly dynamic biomolecular condensates, a behavior important for numerous cellular processes. Here, we present a single-molecule imaging-based method for quantifying the dynamics by which proteins interact with each other in biomolecular condensates in live cells.
Abstract
Biomolecular condensates formed via liquid-liquid phase separation (LLPS) have been considered critical in cellular organization and an increasing number of cellular functions. Characterizing LLPS in live cells is also important because aberrant condensation has been linked to numerous diseases, including cancers and neurodegenerative disorders. LLPS is often driven by selective, transient, and multivalent interactions between intrinsically disordered proteins. Of great interest are the interaction dynamics of proteins participating in LLPS, which are well-summarized by measurements of their binding residence time (RT), that is, the amount of time they spend bound within condensates. Here, we present a method based on live-cell single-molecule imaging that allows us to measure the mean RT of a specific protein within condensates. We simultaneously visualize individual protein molecules and the condensates with which they associate, use single-particle tracking (SPT) to plot single-molecule trajectories, and then fit the trajectories to a model of protein-droplet binding to extract the mean RT of the protein. Finally, we show representative results where this single-molecule imaging method was applied to compare the mean RTs of a protein at its LLPS condensates when fused and unfused to an oligomerizing domain. This protocol is broadly applicable to measuring the interaction dynamics of any protein that participates in LLPS.
Introduction
A growing body of work suggests that biomolecular condensates play an important role in cellular organization and numerous cellular functions, e.g., transcriptional regulation1,2,3,4,5, DNA damage repair6,7,8, chromatin organization9,10,11,12, X-chromosome inactivation13,14,15, and intracellular signaling16,17,18. In addition, the dysregulation of biomolecular condensates is implicated in many diseases, including cancers19,20,21 and neurodegenerative disorders22,23,24,25,26. Condensate formation is often driven by transient, selective, and multivalent protein-protein, protein-nucleic acid, or nucleic acid-nucleic acid interactions27. Under certain conditions, these interactions can lead to liquid-liquid phase separation (LLPS), a density transition that locally enriches specific biomolecules in membraneless droplets. Such multivalent interactions are often mediated by the intrinsically disordered regions (IDRs) of proteins1,28,29. Biophysical characterization of these interactions at the molecular level is critical to our understanding of numerous healthy and aberrant cellular functions, given the pervasiveness of condensates across them. Although techniques based on confocal fluorescence microscopy, e.g., fluorescence recovery after photobleaching (FRAP)30,31,32, have been widely used to qualitatively show that the molecular exchanges between condensates and the surrounding cellular environment are dynamic, quantifying the interaction dynamics of specific biomolecules within condensates is generally not possible using conventional confocal microscopy or single-molecule microscopy without specialized data analysis methods. The single-particle tracking (SPT) technique described in this protocol is based on live-cell single-molecule microscopy33 and provides a uniquely powerful tool to quantify the interaction dynamics between specific proteins within condensates. The readout of SPT for such measurement is the mean residence time of a protein of interest in the condensates.
The protocol can be broken down into two parts – data acquisition and data analysis. The first step of imaging data acquisition is to express in cells a protein of interest that is fused to a HaloTag34. This enables labeling of the protein of interest with two fluorophores, where a majority of the protein molecules are to be labeled with a non-photoactivatable fluorophore (e.g., JFX549 Halo ligand35) and a small fraction of them are to be labeled with a spectrally distinct, photoactivatable fluorophore (e.g., PA-JF646 Halo ligand36). This allows for the simultaneous acquisition of all condensate locations in the cell and the acquisition of single-molecule movies of the protein of interest binding and unbinding to the condensates. Meanwhile, the same type of cells are modified to stably express Halo-tagged H2B, a histone that is largely immobile on chromatin. The cells are then stained with the PA-JF646 Halo ligand to enable single-molecule imaging of H2B. As will be discussed in detail below, this experiment accounts for the contribution of photobleaching to enable precise quantification of the interaction dynamics of the protein of interest. Cells for imaging experiments must then be cultured on clean coverslips, stained with HaloTag ligand(s), and assembled into a live-cell imaging chamber. From there, the sample is imaged under highly inclined and laminated optical sheet (HILO) illumination on a total internal reflection fluorescence (TIRF) microscope capable of two-channel imaging and single-molecule detection. The emission is then split onto two cameras, one tracking condensate positions and one tracking single molecules. Acquisition is performed with a long integration time (on the order of hundreds of ms) to blur out freely-diffusing proteins and only capture proteins that are less mobile due to binding to stable structures in the cell37.
The first step of data analysis is using an established single-particle tracking (SPT) algorithm38,39 to localize individual protein molecules in each frame of the movie and assemble the localizations into a trajectory for each molecule over its detectable lifetime. The trajectories are then sorted into those representing molecules inside and those representing molecules outside the condensates by comparing the localizations of the molecules throughout their trajectories to the localizations of all the condensates at the corresponding times1.
Next, a survival curve (1 – CDF) is generated using the lengths of all the in-condensate trajectories. The apparent mean residence time of the molecules is then extracted by fitting the survival curve to the following two-component exponential model of protein binding,
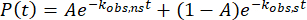 ,
,
with A as the fraction of molecules non-specifically bound and with kobs,ns and kobs,s as the observed dissociation rates of the non-specifically bound and specifically bound molecules, respectively. Only kobs,s is considered from here onward. The dynamics of both protein dissociation, ktrue,s, and photobleaching of the fluorophore, kpb, contribute to kobs,s as
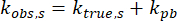 ;
;
thus, to isolate the effects of protein dissociation, the specific dissociation rate of H2B-Halo in the cell line mentioned prior is measured.
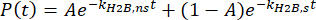
H2B is a protein that is stably integrated into chromatin and that experiences minimal dissociation in the time scale of a single-molecule movie acquisition37. Its specific dissociation rate is then equal to the photobleaching rate of the PA-JF646 Halo ligand, or
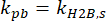 .
.
The mean in-condensate residence time of the protein of interest, 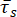 , is then
, is then
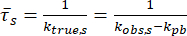 .
.
Representative results from Irgen-Gioro et al.40 are shown, where this protocol was applied to demonstrate that fusing an oligomerization domain to IDR results in longer residence times of the IDR in its condensates. This result suggests that the added oligomerization domain stabilizes the homotypic interactions of the IDR that drives LLPS. In principle, the same method with slightly modified protocols can be applied to characterize the homotypic or heterotypic interactions of any protein that participates in the formation of any types of condensates.
Protocol
Representative Results
Discussion
The protocol as presented here is designed for systems like those investigated in Irgen-Gioro et al.40. Depending on the application, some components of the protocol can be modified, e.g., the method for generating fluorescently labeled cell lines, the fluorescent labeling system, and the style of coverslip used. Halo-tagging of a protein in a cell can be done using two strategies, depending on which is more suitable for a given experiment. 1) Exogenous expression: fusing the protein of interest t…
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1745301 (S.Y.), Pew-Stewart Scholar Award (S.C.), Searle Scholar Award (S.C.), the Shurl and Kay Curci Foundation Research Grant (S.C.), Merkin Innovation Seed Grant (S.C.), the Mallinckrodt Research Grant (S.C.), and the Margaret E. Early Medical Research Trust 2024 Grant (S.C.). S.C. is also supported by the NIH/NCI under Award Number P30CA016042.
Materials
| 0.1 µm TetraSpeck microsphere | Invitrogen | T7279 | Single-molecule imaging |
| 25 mm Diameter, #1.5 Coverslips | Marienfeld Superior | 111650 | Preparation of coverslips |
| 593/40 nm bandpass filter | Semrock | FF01-593/40-25 | Single-molecule imaging |
| 676/37 nm bandpass filter | Semrock | FF01-676/37-25 | Single-molecule imaging |
| 6-Well TC Plate | Genesee | 25-105MP | Preparation of cells for microscopy |
| Cell Line: U-2 OS | ATCC | HTB-96 | Labeling of proteins in cells |
| ConvertASCII_SlowTracking_css3 .m |
Analysis of single-molecule imaging data: Available in Chong et al., 2018 | ||
| Coverglass Staining Rack | Thomas | 24957 | Preparation of coverslips |
| Deuterated Janelia Fluor 549 (JFX549) | Janelia Research Campus | Preparation of cells for microscopy | |
| DMEM, Low Glucose | Gibco | 10-567-022 | Labeling of proteins in cells: Growth media used: DMEM with 5% fetal bovine serum, 1% penstrep |
| Eclipse Ti2-E Inverted Microscope | Nikon | Single-molecule imaging | |
| Ethanol 200 Proof | Lab Alley | EAP200-1GAL | Preparation of coverslips |
| evalSPT | Analysis of single-molecule imaging data: Available in Drosopoulos et al., 2020 | ||
| Fetal Bovine Serum | Cytiva | SH30396.03 | Labeling of proteins in cells: Growth media used: DMEM with 5% fetal bovine serum, 1% penstrep |
| Fiji | Analysis of single-molecule imaging data | ||
| Ikon Ultra CCD Camera | Andor | X-13723 | Single-molecule imaging |
| Longpass dichroic beamsplitter | Semrock | Di02-R635-25×36 | Single-molecule imaging: Red/Far Red beamsplitter |
| LUN-F Laser Unit | Nikon | Single-molecule imaging: 405/488/561/640 | |
| MatTek glass-bottom dish | MatTek | P35G-1.5-20-C | Preparation of cells for microscopy: 35 mm, #1.5 coverslip dish for cell culture. |
| NIS-Elements | Nikon | Single-molecule imaging: Microscope acquisition software | |
| nucleus and cluster mask_v2.txt | Analysis of single-molecule imaging data: Available in Chong et al., 2018 | ||
| Penicillin-Streptomycin | Gibco | 15-140-122 | Labeling of proteins in cells: Growth media used: DMEM with 5% fetal bovine serum, 1% penstrep |
| Phosphate Buffered Saline | Thermo Fisher Scientific | 18912014 | Labeling of proteins in cells |
| Photoactivatable Janelia Fluor 646 (PA-JF646) | Janelia Research Campus | Preparation of cells for microscopy | |
| PLOT_ResidenceHist_css.m | Analysis of single-molecule imaging data: Available in Chong et al., 2018 | ||
| Potassium Hydroxide | Mallinckrodt Chemicals | 6984-06 | Preparation of coverslips |
| pretracking_comb.txt | Analysis of single-molecule imaging data: Available in Chong et al., 2018 | ||
| SLIMfast | Analysis of single-molecule imaging data: Available in Teves et al., 2016 | ||
| Stage-top incubation system | Tokai Hit | Single-molecule imaging: For live-cell imaging | |
| TwinCam dual emission image splitter | Cairn Research | Single-molecule imaging | |
| Ultrasonic Cleaner | Branson | 5800 | Preparation of coverslips |
Referências
- Chong, S., et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science. 361 (6400), (2018).
- Chong, S., Graham, T. G. W., Dugast-Darzacq, C., Dailey, G. M., Darzacq, X., Tjian, R. Tuning levels of low-complexity domain interactions to modulate endogenous oncogenic transcription. Molecular Cell. 82 (11), 2084-2097 (2022).
- Boehning, M., et al. RNA polymerase II clustering through carboxy-terminal domain phase separation. Nature Structural & Molecular Biology. 25 (9), 833-840 (2018).
- Sabari, B. R., et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science. 361 (6400), (2018).
- Boija, A., et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell. 175 (7), 1842-1855 (2018).
- Levone, B. R., et al. FUS-dependent liquid-liquid phase separation is important for DNA repair initiation. Journal of Cell Biology. 220 (5), e202008030 (2021).
- Kilic, S., et al. Phase separation of 53BP1 determines liquid-like behavior of DNA repair compartments. The EMBO Journal. 38 (16), e101379 (2019).
- Pessina, F., et al. Functional transcription promoters at DNA double-strand breaks mediate RNA-driven phase separation of damage-response factors. Nature Cell Biology. 21 (10), 1286-1299 (2019).
- Nozaki, T., et al. Condensed but liquid-like domain organization of active chromatin regions in living human cells. Science Advances. 9 (14), (2023).
- Maeshima, K., et al. Nucleosomal arrays self-assemble into supramolecular globular structures lacking 30-nm fibers. The EMBO Journal. 35 (10), 1115-1132 (2016).
- Strickfaden, H., Tolsma, T. O., Sharma, A., Underhill, D. A., Hansen, J. C., Hendzel, M. J. Condensed chromatin behaves like a solid on the mesoscale in vitro and in living cells. Cell. 183 (7), 1772-1784 (2020).
- Gibson, B. A., et al. Organization of chromatin by intrinsic and regulated phase separation. Cell. 179 (2), 470-484 (2019).
- Cerase, A., Armaos, A., Neumayer, C., Avner, P., Guttman, M., Tartaglia, G. G. Phase separation drives X-chromosome inactivation: a hypothesis. Nature Structural & Molecular Biology. 26 (5), 331-334 (2019).
- Jachowicz, J. W., Strehle, M., Banerjee, A. K., Blanco, M. R., Thai, J., Guttman, M. Xist spatially amplifies SHARP/SPEN recruitment to balance chromosome-wide silencing and specificity to the X chromosome. Nature Structural & Molecular Biology. 29 (3), 239-249 (2022).
- Pandya-Jones, A., et al. A protein assembly mediates Xist localization and gene silencing. Nature. 587 (7832), 145-151 (2020).
- Du, M., Chen, Z. J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 361 (6403), 704-709 (2018).
- Zamudio, A. V., et al. Mediator condensates localize signaling factors to key cell identity genes. Molecular Cell. 76 (5), 753-766 (2019).
- Zhang, J. Z., et al. Phase separation of a PKA regulatory subunit controls cAMP compartmentation and oncogenic signaling. Cell. 182 (6), 1531-1544 (2020).
- Kovar, H. Dr. Jekyll and Mr. Hyde: The two faces of the FUS/EWS/TAF15 protein family. Sarcoma. 2011, e837474 (2010).
- Linardic, C. M. PAX3-FOXO1 fusion gene in rhabdomyosarcoma. Cancer Letters. 270 (1), 10-18 (2008).
- Ahn, J. H., et al. Phase separation drives aberrant chromatin looping and cancer development. Nature. 595 (7868), 591-595 (2021).
- Wegmann, S., et al. Tau protein liquid-liquid phase separation can initiate tau aggregation. The EMBO Journal. 37 (7), e98049 (2018).
- Friedman, M. J., et al. Polyglutamine domain modulates the TBP-TFIIB interaction: implications for its normal function and neurodegeneration. Nature Neuroscience. 10 (12), 1519-1528 (2007).
- Molliex, A., et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 163 (1), 123-133 (2015).
- Murakami, T., et al. ALS/FTD mutation-induced phase transition of FUS liquid Droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron. 88 (4), 678-690 (2015).
- Patel, A., et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 162 (5), 1066-1077 (2015).
- Shin, Y., Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science. 357 (6357), (2017).
- Kato, M., McKnight, S. L. A solid-state conceptualization of information transfer from gene to message to protein. Annual Review of Biochemistry. 87, 351-390 (2018).
- Li, P., et al. Phase transitions in the assembly of multivalent signalling proteins. Nature. 483 (7389), 336-340 (2012).
- Muzzopappa, F., et al. Detecting and quantifying liquid-liquid phase separation in living cells by model-free calibrated half-bleaching. Nature Communications. 13 (1), 7787 (2022).
- Sprague, B. L., Müller, F., Pego, R. L., Bungay, P. M., Stavreva, D. A., McNally, J. G. Analysis of binding at a single spatially localized cluster of binding sites by fluorescence recovery after photobleaching. Biophysical Journal. 91 (4), 1169-1191 (2006).
- Taylor, N. O., Wei, M. -. T., Stone, H. A., Brangwynne, C. P. Quantifying dynamics in phase-separated condensates using fluorescence recovery after photobleaching. Biophysical Journal. 117 (7), 1285-1300 (2019).
- Liu, Z., Lavis, L. D., Betzig, E. Imaging live-cell dynamics and structure at the single-molecule level. Molecular Cell. 58 (4), 644-659 (2015).
- Los, G. V., et al. HaloTag: A novel protein labeling technology for cell imaging and protein analysis. ACS Chemical Biology. 3 (6), 373-382 (2008).
- Grimm, J. B., et al. A general method to improve fluorophores using deuterated auxochromes. JACS Au. 1 (5), 690-696 (2021).
- Grimm, J. B., et al. photoactivatable fluorophores for single-molecule imaging. Nature Methods. 13 (12), 985-988 (2016).
- Hansen, A. S., Pustova, I., Cattoglio, C., Tjian, R., Darzacq, X. CTCF and cohesin regulate chromatin loop stability with distinct dynamics. eLife. 6, 25776 (2017).
- Sergé, A., Bertaux, N., Rigneault, H., Marguet, D. Dynamic multiple-target tracing to probe spatiotemporal cartography of cell membranes. Nature Methods. 5 (8), 687-694 (2008).
- Normanno, D., et al. Probing the target search of DNA-binding proteins in mammalian cells using TetR as model searcher. Nature Communications. 6 (1), 7357 (2015).
- Irgen-Gioro, S., Yoshida, S., Walling, V., Chong, S. Fixation can change the appearance of phase separation in living cells. eLife. 11, e79903 (2022).
- Tokunaga, M., Imamoto, N., Sakata-Sogawa, K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nature Methods. 5 (2), 159-161 (2008).
- Teves, S. S., An, L., Hansen, A. S., Xie, L., Darzacq, X., Tjian, R. A dynamic mode of mitotic bookmarking by transcription factors. eLife. 5, e22280 (2016).
- Drosopoulos, W. C., Vierra, D. A., Kenworthy, C. A., Coleman, R. A., Schildkraut, C. L. Dynamic assembly and disassembly of the human DNA polymerase δ holoenzyme on the genome In vivo. Cell Reports. 30 (5), 1329 (2020).
- Cong, L., et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 339 (6121), 819-823 (2013).
- Yoshida, S. R., Maity, B. K., Chong, S. Visualizing protein localizations in fixed cells: Caveats and the underlying mechanisms. The Journal of Physical Chemistry B. 127 (19), 4165-4173 (2023).
- Gautier, A., et al. An engineered protein tag for multiprotein labeling in living cells. Chemistry & Biology. 15 (2), 128-136 (2008).
- Hipp, L., et al. Single-molecule imaging of the transcription factor SRF reveals prolonged chromatin-binding kinetics upon cell stimulation. Proceedings of the National Academy of Sciences. 116 (3), 880-889 (2019).
- Agarwal, H., Reisser, M., Wortmann, C., Gebhardt, J. C. M. Direct observation of cell-cycle-dependent interactions between CTCF and chromatin. Biophysical Journal. 112 (10), 2051-2055 (2017).
- Chen, L., Zhang, Z., Han, Q., Maity, B. K., Rodrigues, L., Zboril, E., Adhikari, R., Ko, S. H., Li, X., Yoshida, S. R., Xue, P., Smith, E., Xu, K., Wang, Q., Huang, T. H., Chong, S., Liu, Z. Hormone-induced enhancer assembly requires an optimal level of hormone receptor multivalent interactions. Molecular cell. 83 (19), 3438-3456 (2023).
- Garcia, D. A., et al. Power-law behavior of transcription factor dynamics at the single-molecule level implies a continuum affinity model. Nucleic Acids Research. 49 (12), 6605-6620 (2021).

