Engineering Intracellular Protein Sensors in Mammalian Cells
Summary
Here, we present a protocol for engineering genetically-encoded intracellular protein sensor-actuator(s). The device specifically detects target proteins through intracellular antibodies (intrabodies) and responds by switching on gene transcriptional output. A general framework is built to rapidly replace intrabodies, enabling rapid detection of any desired protein, without altering the general architecture.
Abstract
Proteins can function as biomarkers of pathological conditions, such as neurodegenerative diseases, infections or metabolic syndromes. Engineering cells to sense and respond to these biomarkers may help the understanding of molecular mechanisms underlying pathologies, as well as to develop new cell-based therapies. While several systems that detect extracellular proteins have been developed, a modular framework that can be easily re-engineered to sense different intracellular proteins was missing.
Here, we describe a protocol to implement a modular genetic platform that senses intracellular proteins and activates a specific cellular response. The device operates on intracellular antibodies or small peptides to sense with high specificity the protein of interest, triggering the transcriptional activation of output genes, through a TEV protease (TEVp)-based actuation module. TEVp is a viral protease that selectively cleaves short cognate peptides and is widely used in biotechnology and synthetic biology for its high orthogonality to the cleavage site. Specifically, we engineered devices that recognize and respond to protein-biomarkers of viral infections and genetic diseases, including mutated huntingtin, NS3 serine-protease, Tat and Nef proteins to detect Huntington’s disease, hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infections, respectively. Importantly, the system can be hand tailored for the desired input-output functional outcome, such as fluorescent readouts for biosensors, stimulation of antigen presentation for immune response, or initiation of apoptosis to eliminate unhealthy cells.
Introduction
The study and modulations of cellular responses via controllable engineered gene circuits are major goals in synthetic biology1,2,3 for the development of prospective tools with relevant biological or medical applications in cancer4, infections5, metabolic diseases6, and immunology7.
Reprogramming cell functions in response to specific signals requires the design of smart interfaces that link sensing of extracellular or intracellular dynamic changes (input) to downstream processing, triggering specific output either for diagnostic purposes (i.e., reporter genes) or to rewire cell response (therapeutics). The inputs detected by the sensing module can be small analytes8, proteins9,10,11 or microRNAs11,12,13, specific for the onset or progression of a disease. Moreover, complex circuit regulation can be achieved by multiple input information processing, increasing the tight control over transgene expression in response to the defined conditions14,15,16. For example, microRNA-based sensors can identify specific cell types, such as cancer cells, inducing their clearance with the expression of an apoptotic gene13. Since microRNAs are easily implementable in synthetic circuits in a modular manner, they represent a widely used input for genetically encoded biosensors12,13,17. Proteins are also a valid biomarker for genetic mutations, cancer and infections, and indeed a number of extracellular protein-sensing devices have been reported18,19.
Many of the circuits that detect extracellular proteins rely on the use of engineered receptors, which tether a transcription factor (TF) to the membrane, fused to a TEVp-responsive cleavage site (TCS). A major advantage of the TEVp is the specificity of the cleavage and lack of interference with endogenous protein processing. In these systems, TEVp is fused to a second peptide that interacts with the engineered receptor upon binding of the extracellular molecules. Thus, the external inputs induce TEVp-mediated cleavage and TF release. Systems that function with this mechanism are Tango/TEVp18, light-induced20 and Modular Extracellular Sensor Architecture (MESA)19. Despite progress in detecting extracellular proteins, the technology for sensing intracellular proteins in a modular fashion was never realized before, with the limitation of going through many build-and-test-iterations for single devices responsive to a specific protein.
Our system is the first platform for intracellular protein sensing21. The modularity is guaranteed by the use of intrabodies that define the specificity to the target, whereas the cell-reprogramming is TEVp-mediated. Specifically, one intrabody is membrane bound and fused to the C-terminal to a fluorescent protein mKate, a TCS and a TF (fusion protein 1); the second intrabody is fused to the TEVp and located in the cytosol (fusion protein 2) (Figure 1).
Thus, the interaction between two intrabodies and the target protein occurs in the cytoplasm and leads to TCS cleavage by TEVp, resulting in TF translocation into the nucleus to activate functional output. The sensing—actuating device was successfully tested for four intracellular disease-specific proteins: NS3 serine protease expressed by the HCV virus22, Tat and Nef proteins from HIV infection23,24, and mutated huntingtin (HTT) of the Huntington’s disease25. Output expression includes fluorescent reporters, apoptotic gene (hBax)26 and immunomodulators (XCL-1)27. We demonstrate that the system can also impair pathological functionality of its targets. For instance, the Nef-responsive device interferes with the spreading of viral infection by sequestering the target protein and reverting the downmodulation of HLA-I receptor on infected T cells24. The described sensing-actuating platform is the first of this kind for the detection of intracellular proteins and can be potentially implemented to sense abnormal protein expression, post-translational or epigenetic modifications, for diagnostic and therapeutic purposes20.
Protocol
1. Design principles for construction and test the sensor-actuator device
- Select a protein of interest.
NOTE: We designed a system for proteins located in the cytoplasm or shuttling between the cytoplasm and other compartments. - Select two intrabodies binding different epitopes of the target protein. In our study we selected proteins for which the intrabodies were already developed and tested28,29,30,31. When intrabodies are not available, they should be newly developed.
NOTE: An alternative to intrabodies can be other molecules that interact with the protein of interest. For example, the Nef sensing device include one intrabody (sdAb19) and the SH3 domain of a p59fyn protein tyrosine kinase32. - Design in silico the plasmids with DNA sequences encoding the fusion proteins.
NOTE: Software for plasmid design are available for free or can be purchased.- Generate sequences that include the following modules: membrane tag, fluorescent marker (e.g., mKate), intrabody, TCS and TF for fusion protein 1, and intrabody and TEVp for fusion protein 2.
NOTE: Building a new architecture based on the interaction of several components requires attention to parameters that may maximize the signal activation when the target protein is detected. Specifically, we focused on TEVp activity and flexibility of the chimeric proteins to facilitate protein-protein interaction (Table 1). All these features are specified in the following points. - Insert a flexible glycine-serine linker domain (LD) between the intrabody–TCS and/or TEVp–intrabody. The LD may be of variable length (0, 10 or 15 amino acids-AA).
- Fusion protein 1: Design variants that include the high affinity TCS (S) or the low affinity TCS (L) to regulate TEVp cleavage activity. The expression of this construct is driven by a constitutive promoter. Include the GAL4VP16 transcription factor at the C-terminal of TCS to allow translocation to the nucleus upon TEVp cleavage.
NOTE: We used the hEF1a promoter, but other constitutive promoters can be chosen. - Fusion protein 2: Use a constitutive (hEF1a) or doxyclycine-inducible (pTET) promoter to tune TEVp transcription. Use a degradation domain (DD) regulated by the small molecule shield to modulate protein stability.
NOTE: Other inducible systems that tune protein expression can potentially be chosen. - Output: Place a reporter gene downstream UAS promoter that respond to GAL4VP16 transcription factor. Choose the output according to the application of the device (i.e., fluorescent protein, cytokine, etc.).
- Generate sequences that include the following modules: membrane tag, fluorescent marker (e.g., mKate), intrabody, TCS and TF for fusion protein 1, and intrabody and TEVp for fusion protein 2.
- Perform cloning with any preferred strategy. Here, we built plasmids with golden gate or gateway technologies.
- Test the various configuration of the device in the desired cell lines by transient transfection. Co-transfect the cells with the obtained plasmids for fusion proteins 1 and 2 and fluorescent reporter gene, such as UAS-EYFP. Fusion protein 1 include a red fluorescent protein (mkate) used as transfection marker.
NOTE: Start with simple-to-transfect cell lines such as HEK293FT cells to test the devices.- Transfection in HEK293FT cells. Seed 2 x 105 cells into a 24-well plate with 500 µL of DMEM. Transfect cells with 300 ng of total DNA using transfection reagent, vortex the mix and incubate at RT for 20 minutes.
- Place cells in a 37 °C/5% CO2 incubator. Add fresh culture medium 24 h after transfection.
- Transfection in Jurkat cells. Use 3 x 105 cells in 500 µL of RPMI cell culture medium for 24-well plate. Perform electroporation following manufacturer’s instruction. Use 2-4 µg of total DNA for each sample. Add 500 µL of fresh cell culture media 24 h after transfection.
- During electroporation, place cells and DNA mix on ice. Then, place the cells in the 37 °C/5% CO2 incubator.
NOTE: Different cell lines and transfection methods may be chosen according to the specific application of the device.
- During electroporation, place cells and DNA mix on ice. Then, place the cells in the 37 °C/5% CO2 incubator.
- Transfection in HEK293FT cells. Seed 2 x 105 cells into a 24-well plate with 500 µL of DMEM. Transfect cells with 300 ng of total DNA using transfection reagent, vortex the mix and incubate at RT for 20 minutes.
- Acquire microscope images to obtain a qualitative understanding of the functionality of the device. Confirm membrane localization of the fusion protein 1 by detecting the fluorescent signal (mkate) in proximity to the cell membrane. Visualize the fluorescent output (EYFP) in the presence or absence (negative control-NC) of the target protein.
- Use the cell imaging system equipped with Tx Red and GFP light cubes to detect mKate and EYFP fluorescent proteins. Use the 10x or 20x objective.
NOTE: Use non-transfected cells to ensure that there is no autofluorescence.
- Use the cell imaging system equipped with Tx Red and GFP light cubes to detect mKate and EYFP fluorescent proteins. Use the 10x or 20x objective.
- Analyze the transfection with flow cytometry (FACS).
NOTE: See Protocol 2 for sample preparation.- Evaluate the devices in the presence of the target protein. Create an experimental matrix that combine variants of fusion protein 1 and 2, modulating TEVp with (i) constitutive hEf1a promoter (ii) doxycycline-inducible TET promoter and (iii) shield for protein stability. Add doxycycline and shield at concentrations (0-1000 nM).
- Perform FACS analysis to compare the fold induction of EYFP between samples with or without protein of interest.
NOTE: The highest fold induction corresponds to the greatest input sensitivity in ON (presence of the target protein) as compared to the OFF (absence of the target protein) mode.
- Test the functionality of the device with respect to the desired applications.
- Run FACS analysis to measure the levels of output proteins or apoptosis, or other relevant assays.
- Perform RT PCR analysis if the output part of the device regulates expression levels of target genes. Calculate 2-ddCT in respect to the house-keeping gene (GAPDH or equivalent) and compare the fold change of output expression in ON and OFF modes.
2. Sample preparation for flow cytometry analysis
- Perform quantitative analysis of protein devices with flow cytometer (FACS) 48 h after transfection.
- Aspirate the DMEM media and wash HEK293FT cells with 300 µL of PBS. Add 100 µL of trypsin and leave in the 37 °C/5% CO2 incubator for 2-4 minutes.
NOTE: Jurkat cells do not require trypsin. - Add 400 µL of DMEM media without phenol red and resuspend the cells to avoid clumps formation. Transfer in FACS tubes.
- Use a flow cytometer equipped with 405, 488, and 561 nm lasers.
- Collect 30,000 – 100,000 events selected from live cell population using the following cytometer settings: 488 nm laser and 530/30 nm bandpass filter for EYFP/EGFP, 561 nm laser and 610/20 nm filter for mKate, and 405 nm laser, 450/50 filter for EBFP.
NOTE: To set up the flow cytometer, include non-transfected cells and cells transfected with single fluorescent proteins. - Perform analysis of collected samples using preferred software (e.g., DIVA software, Flowjo, or TASBE33 method).
- Aspirate the DMEM media and wash HEK293FT cells with 300 µL of PBS. Add 100 µL of trypsin and leave in the 37 °C/5% CO2 incubator for 2-4 minutes.
3. Characterization of the HCV sensor-actuator device
- Select intrabodies to detect NS3 protein. scFv35 and scFv162 bind to two different epitopes and were thus chosen.
- Design and test variants of the NS3-responsive device following the rational described in step 1.3 (e.g., Fusion protein 1: membrane tag, mKate, single chain fragment intrabody scFv35, LD0, TCS-L and TF GAL4VP16; Fusion protein 2: single chain fragment intrabody scFv162, LD0 and TEVp; Output: UAS-EYFP or UAS-hBax (apoptosis); NS3 (see Table 2)).
- Test the constructs in HEK293FT cells.
NOTE: For each experiment, set a negative control that is the delivery of the device and output quantification in the absence of the protein of interest.- Test the devices for TEVp under a constitutive promoter in the presence or absence of NS3. Perform transfection of fusion protein 1 (step 1.3.3), fusion protein 2 (step 1.3.4) and EYFP output step (1.3.5) in HEK293FT cells as described in step 1.5.1. Analyze the samples 48 h post-transfection by measuring the fluorescent reporter (EYFP) by flow cytometry (Protocol 2).
- Assess the interaction intrabody-NS3 by mKate and BFP colocalization using the BFP-NS3 fusion protein. Transfect cells with fusion protein 1 that includes mKate, and with a BFP-NS3. 48 h post-transfection, observe cells at confocal microscope (63x objective) to test for co-localization of fluorescent proteins.
NOTE: Use a BFP (not fused to NS3) as negative control. In this condition BFP should show diffuse cellular localization and should not co-localize with the membrane-bound mKate. - Test the devices for TEVp under the pTET promoter in the presence or absence of NS3. Compare EYFP in absence and presence of NS3. Co-transfect cells with fusion protein 1 (step 1.3.3), fusion protein 2 (step 1.3.4) and EYFP output (step 1.3.5) using the protocol described in step 1.5.1. Transfections are performed in the presence or absence of NS3. Analyze the samples 48 h post-transfection by measuring the fluorescent reporter (EYFP) by flow cytometry (Protocol 2).
- Test cell killing driven by NS3 device.
- Replace EYFP output with the apoptotic gene hBax.
- Repeat step 3.3.3. Collect the cells 48 h after transfection and wash with PBS before starting the assay.
- Perform apoptosis assay with Annexin V conjugated to Pacific Blue. Stain cells with apoptotic marker Annexin V (conjugated with 2.5 µL of Pacific Blue) diluted in binding buffer (1:20 volume ratio) for 10 min at room temperature.
- Analyze the cells by flow cytometry. Calculate the apoptosis level within population as number of Pacific-Blue (Annexin V) positive cells.
4. Characterization of Nef-responsive HIV sensor-actuator device
- Select intrabodies that bind to Nef. Single domain intrabody sdAb19 and SH3 were chosen as they bind to different epitopes.
NOTE: SH3 is not an intrabody but a domain of p59fyn protein tyrosine kinase that physiologically interacts with Nef. - Design and test variants of Nef-responsive device following the rational described in step 1.3 (e.g., Fusion protein 1: membrane tag, mKate, sdAb19, LD0, TCS-L and GAL4-VP16; Fusion protein 2: SH3, LD0 and TEVp; Output: UAS-EYFP; Nef (see Table 3)).
- Test the devices in HEK293FT and Jurkat T cells.
NOTE: For each experiment we set a negative control that is delivery of the device and output quantification in the absence of the protein of interest- Transfection of HEK293FT: Follow the procedure described in step 1.5.1 in presence or absence of Nef.
- Transfection of Jurkat T cells: Follow the procedure described in step 1.5.2 in presence or absence of Nef.
- 48h post-transfection, analyze the samples with flow cytometer as described in Protocol 2.
- Infect Jurkat cells with HIV LAI strains for HIV detection.
- Prepare viral stocks at least 48 h before the experiment. Use virus obtained from transfection of HEK-293T cells with the infectious molecular clones (NIH AIDS reagents program) and commercial reagents using manufacturer instructions. Concentrate virus after 40 h by ultracentrifugation for 1 h, 64,074 x g, 4°C on 20% sucrose to avoid viral particle-free proteins. Titer viral stocks with HIV-1 p24 ELISA.
- Infect cells with HIV-1 strains with 500 ng of p24 inoculum. 40 h post-infection quantify the percentage of infected cells by detecting the viral protein p24 with KC57-FITC conjugated antibody and performing flow-cytometry analysis.
- Stain the cells with AlexaFluor 647 conjugated antibodies clone W6/32 against HLA-A, B, C. Fix the cells and analyze on a flow cytometer. Nef mediated downmodulation of HLA-I in Jurkat T cells in the presence or absence of the protein sensor device.
Representative Results
An architecture for modular intracellular protein detection
As shown in Figure 1, the device is composed of: 1) intrabody 1 connected to the membrane-tethered fluorescent marker (mKate) and TEVp cleavage site (TCS), followed by a transcription activator GAL4VP16 (TF); 2) intrabody 2 fused to TEV protease (TEVp), free in the cytosol; 3) a synthetic promoter responsive to GAL4VP16, driving the expression of a reporter gene. The modularity is guaranteed by intrabodies that can be changed in a plug-and-play manner to target any desired protein. Also, the output can be chosen depending on the desired application. A critical aspect in order to optimize sensing-actuation performance is to avoid background activation of the system (in absence of the protein of interest). We thus tuned several features of the device (Table 1).
HCV, Huntingtin and HIV sensor-actuator devices
The first device was built to recognize NS3 protein, expressed from the epatitis C virus HCV29. The study of this sensor set the rules for the following ones. In specific, we co-transfected fusion protein 1 variants of the NS3-responsive devices along with constitutively expressed (hEF1α) TEVp-scFv162 in HEK293FT cells. We observed NS3-independent output activation both with the TCS(S) and TCS(L) (Figure 2a), indicating that the system requires fine regulation of TEVp activity. To achieve input specific signal activation, we modulated TEVp expression transcriptionally and post-translationally. The first was mediated by a doxycycline (dox) responsive promoter (pTET), whereas the second was obtained by adding to TEVp a degradation domain tag (DD degron) regulated by the small molecule shield. The device was able to specifically induce gene expression following NS3 detection, in the absence of dox and shield when the system included the TCS(L) (Figure 2b). These results indicate that minimal TEVp concentration is required for efficient devices. On the contrary maximal activation of TEVp by dox and shield in the TCS(L) configuration, or the TCS(S) exhibits high background (Figure 2b, c). Altogether, the results demonstrate that the simple leakiness of the promoter (absence of doxycycline or the activator rtTA3) enables the best ON/OFF condition, highlighting the high sensitivity of the TEVp actuator.
Based on the results we designed further NS3 device variants by changing parameters previously mentioned (Table 1) and demonstrated up to 24 EYFP fold induction (Figure 2d,f), and specific cell killing (data in20). Further, we fused the NS3 to a blue fluorescent protein and demonstrated colocalization with fusion protein 1, indicating the interaction between the component of the system (Figure 2e).
Nef sensor-actuator device
Differently from the previous example, the sensing module of Nef device is composed of a single domain antibody (sdAb19)31, and SH3 domain from p59fyn protein tyrosine kinase that is highly expressed in T lymphocytes and implicated in antigen induced T-cell activation34. Since Nef is involved in HLA-I downmodulation, we hypothesized that by sequestering Nef, the device could impair its pathological activity (depicted in Figure 3).
We first tested the Nef-responsive device in 293FT HEK and Jurkat T cells. The sensor exhibited up to 5-fold induction of EYFP in the presence of the target protein (Figure 4a). Different from the NS3 case, here the activation of TEVp by dox (D100) was required to obtain a functional device, whereas LD0 demonstrated lower background in the absence of the target protein. Moreover, we observed EYFP expression in Jurkat T cells infected with X4 LAI strain (Figure 4b). We also demonstrate that the device impairs Nef-mediated downregulation of HLA-I (Figure 4c). Finally, we rewired the output to induce XCL-1, a chemokine normally secreted by CD8+ T cells to induce a localized CTL response, demonstrating the potential towards selective activation of the immunomodulator (Figure 4d).
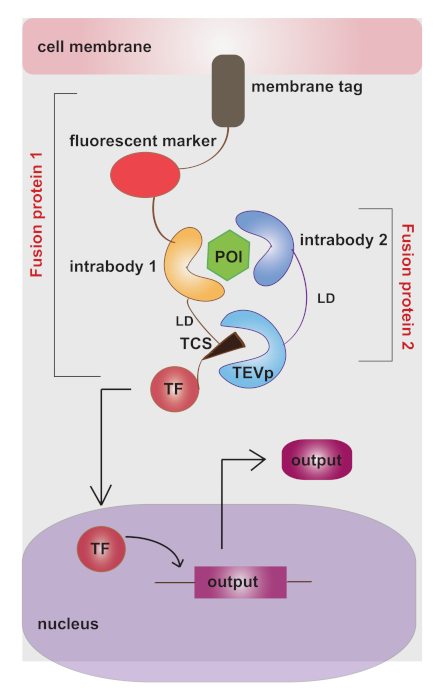
Figure 1: Architecture of the sensor-actuator device for intracellular protein detection. The device is composed of intrabodies (or small peptides) to recognize with high specificity the protein of interest (POI). Interaction of intrabodies with POI brings TEVp in the proximity of the cleavage site (TCS). The membrane-tethered transcription factor (TF) fused to TCS translocates to the nucleus upon TEVp cleavage, activating programmed output. The figure has been modified from20. Please click here to view a larger version of this figure.
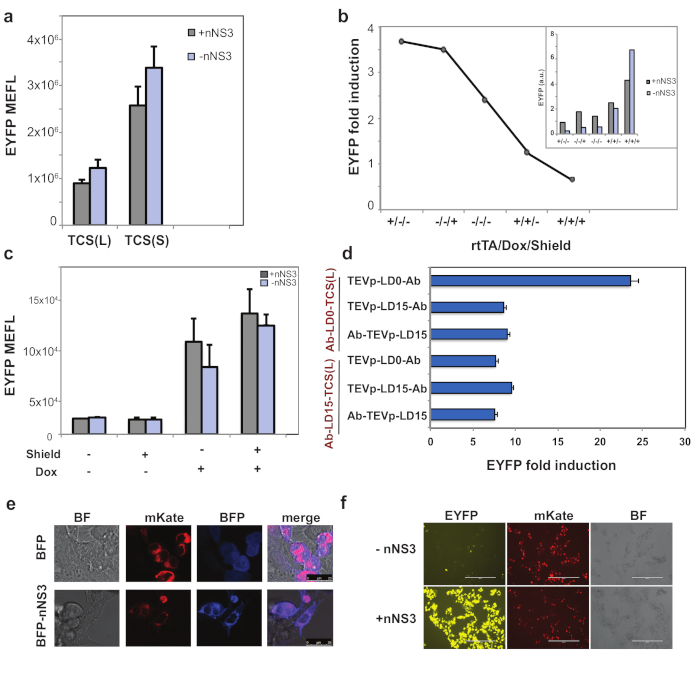
Figure 2: Test of the NS3 device. (a) TCS(S) and (L) variants of NS3 device were tested in HEK293FT cells in presence or absence on NS3. TEVp is driven by a constitutive promoter. Data show that the system is not sensitive to the presence of NS3 due to an overactivation of TEVp that leads to non-specific cleavage of the TCS. (b-c) NS3 induced EYFP reporter activation with TCS(L) but not TCS(S) in absence of Dox and Shield. (d) Test of the variants of NS3 sensor in HEK293FT cells show up to 24x induction of reporter gene when the HVC protein was detected. Data shows fold induction and standard deviation. (e) Co-localization in HEK293FT cells of NS3 fused to a BFP (BFP-nNS3) and fusion protein 1 which include the membrane tethered mKate. As control we used a BFP alone, that shows diffuse cellular localization. Confocal images (scale bar = 25 μm) (f) fluorescent reporter induction as result of NS3 detection in HEK293FT cells. BF: bright field; mKate: fluorescent marker included in fusion protein 1; EYFP: output of the device. (scale bar = 200 μm). The figure has been modified from20. Please click here to view a larger version of this figure.
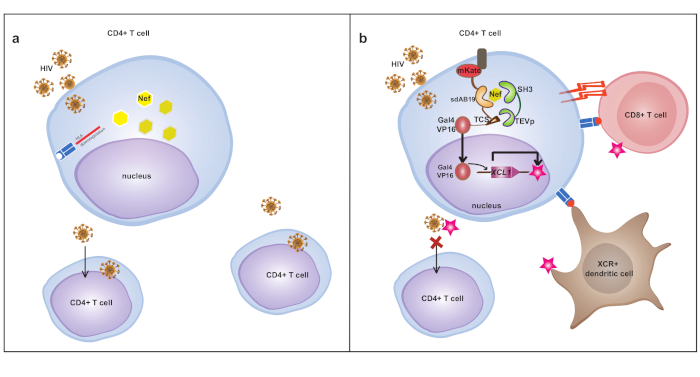
Figure 3: Model of Nef-responsive device. (a) In the absence of the engineered device, HIV escapes the immune response via Nef-mediated HLA downregulation. (b) Nef is detected and sequestered by the sdAb19 intrabody and SH3 domain to activate XCL1 expression. XCL1 stimulates anti-HIV immune response attracting dendritic cells and cytotoxic lymphocytes. In addition, Nef sequestration impairs HLA downmodulation such that CD4+T cells infected by HIV are more responsive to immune system attack. The figure has been modified from20. Please click here to view a larger version of this figure.
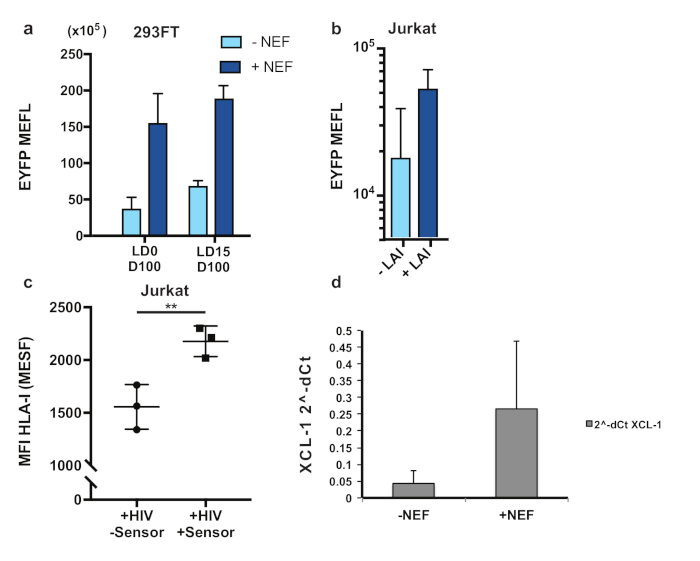
Figure 4: Nef sensor-actuator device detects HIV infection and impair HLA downmodulation. (a) HEK293FT cells induce output activation in the presence of Nef, at intermediate concentration of Dox. (b) Jurkat T cells infected with HIV-LAI strain show reporter activation. (c) Nef device interferes with HLA downmodulation as compared to HLA levels in absence of sensor. (d) XCL-1 immunomodulator is triggered by the device upon Nef detection. The figure has been modified from20. Please click here to view a larger version of this figure.
| 1. Regulation of TEVp expression | |||||
| Transcriptional | Constitutive (hEF1a) | ||||
| Inducible (pTET) | |||||
| Post-translational | Degradation Domain tag (DD degron) + Shield | ||||
| 2. TEV Cleavage Site (TCS) | |||||
| Aa substitutions in P1 | Affinity | ||||
| TCS-S | Serine | High | |||
| TCS-L | Leucine | Low | |||
| 3. Position of TEVp | |||||
| N-terminus of intrabodies | |||||
| C-terminus of intrabodies | |||||
| 4. Glycine-Serine (G4S) Linker Domains (LD) | |||||
| LD0 | 0 Amino acids | ||||
| LD10 | 10 Amino acids | ||||
| LD15 | 15 Amino acids | ||||
| 5. Intrabodies and analogues | |||||
| NS3 | HTT | Tat | Nef | ||
| Intrabody 1 | scFv35 | Happ1 | scFv2 | sdAb19 | |
| Intrabody 2 | scFv162 | VL12.3 | scFv3 | SH3* | |
Table 1: Components for modular device design. Construction of efficient modular devices requires several elements, which can be adjusted to improve the system sensitivity, and specificity. TEVp activity can be adjusted in order to achieve the best signal-to-noise ratio in the system: (1) via transcriptional modulation of TEVp expression using a constitutive (hEF1α) or Doxycycline inducible promoter (pTET), or via posttranslational modulation, using a degradation domain tag (DD) controlled by the small molecule Shield; (2) via high or low affinity TEV cleavage sites (TCS) that include a serine (TCS-S) or a leucine (TCS-L) in P1 position; (3) via repositioning of TEVp to the N or C terminus of the intrabody. To take into account the intrabodies-protein interaction and TEVp accessibility to TCS we (4) included Glycine-Serine (G4S) Linker Domains (LD) of variable amino acid length between intrabody and TCS and between TEVp and intrabody. (5) Selection of intrabodies or their analogues to the target protein. Of note, *SH3 of the Nef-responsive device is not an intrabody but a domain of p59fyn protein tyrosine kinase that bind a proline-rich motif in Nef32. These components were assembled in a plug-and-play fashion to build a modular system towards optimal input-output devices.
| Parameters of the protein sensor-actuator devices | |
| TEVp affinity to the cleavage site | |
| TCS (S) | ENLYFQ S |
| TCS (L) | ENLYFQ L |
| TEVp activity modulation by small molecules | |
| Transcriptional | Doxycycline |
| Translational | Shield |
| TEVp access to the TCS | |
| Fusion protein design | N- or C- terminus fusion o TEVp to the intrabody |
| Protein flexibility | |
| Flexible Linker (LD) | no linker |
| 10 or 15Aa (G4S)3 |
Table 2: Modules of the best NS3 device. Best performing sensory-actuator device is reported for NS3. Precisely, we specify the length of LD domain (LD0, LD15), affinity of the protease binding site TCS (S, L), type of TEVp regulation (hEF1α or pTET, or DD degron).
| Principal engineered modules | ||
| Components | Short name | |
| Nef | Protein of interest | Nef |
| Fusion protein 1 | phEF1a-sdAb19-LD0-TCS(L) | |
| Fusion protein 2 | pTET-TEVp-LD0-SH3 | |
| Output (cytokine) | UAS-XCL1 | |
| Output (fluorescence) | UAS-EYFP | |
Table 3: Modules of the best Nef device. Best performing sensory-actuator device is reported for Nef. Precisely, we specify the length of LD domain (LD0, LD15), affinity of the protease binding site TCS (S, L), type of TEVp regulation (hEF1α or pTET).
Discussion
Until recently, interrogating cells based on intracellular environment was performed with systems developed de novo for specific targets. The present protocol describes an example of the most recent, cell engineering approach for protein sensing and actuating in one device, that can be rapidly adapted to new desired biomarkers.
This pioneering system sense intracellular proteins and provide a specific output to detect or neutralize the disease. The advantage of this class of genetic circuits is the modularity provided by intrabodies and the possibility to use other sensing “parts” to target the protein as shown for the Nef sensor. To our knowledge, a system that can be potentially applied to several intracellular proteins was never demonstrated before.
The modular architecture allows the researchers to adjust the components to maximize ON/OFF ratio of the device for any desired application. This suggests that the device must be carefully tested to achieve the best functional performance. In general, we observed that absence of linker domain (LD0) resulted in more effective device functionality, which perhaps may be the first design strategy for new protein-sensing devices. This is likely due to optimal physical interactions between target protein, intrabodies and TEVp in this device configuration35.
With respect to the actuator module, we observed that TEVp levels, affinity to its cleavage sites, and location in the fusion protein are critical. Constitutive promoters driving protease expression resulted in high background in all devices. In addition, in this experimental set-up TEVp cleavage sites with lower affinity (TCS-L) worked better for NS3, Tat and Nef sensing devices, whereas for HTT detection (reported in20) the TCS-S showed better performances. Last, we observed higher ON/OFF signal ratio with TEVp fused at N-terminal of the intrabody; however, for Tat sensing device TEVp activity was equal at the N- or C-terminal of the chimeric protein.
The described framework allows induction of desired functional response when the protein of interest is detected within the cell. One critical aspect is the sensitivity of the system to the target. We have shown for example that the output expression is a function of NS3 concentration (data shown in20) and that Jurkat T cells were sensitive to HIV infection at titers commonly used in the immunology labs.
Applications of these circuits cover a broad range spanning from dynamic monitoring of cellular state to programmed therapeutic expression upon detection of a disease condition. In perspective, the rapid development of intrabodies with yeast and phage display libraries, that are smaller in size and stable in the cell (i.e. nanobodies) could lead to the design of devices specific for any wanted protein. In addition, protein design tools, and computational models that can predict circuitry performance help in designing effective devices minimizing the build-and-test reiterations.
Our framework provides a novel strategy to connect aberrant protein expression, post-translational modification or viral infection into an information that is translated in transcriptional activation of gene expression.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Istituto Italiano di Tecnologia.
Materials
| AlexaFluor 647 mouse anti-human HLA-A, B, C antibody clone W6/32 | Biolegend | 311414 | Antibodies |
| Annexin V | LifeTechnologies | A35122 | Apoptosis marker |
| Attractene | Qiagen | 301005 | Transfection reagent |
| BD Falcon Round-Bottom Tubes | BD Biosciences | 352053 | FACS tubes |
| Doxycycline | Clonetech | – | Cell Culture: Drugs |
| Dulbecco's modified Eagle medium | Cellgro | 10-013-CM | Cell Culture: Medium |
| Evos Cell Imaging System | Life Technology | EVOS M5000 | Imaging systems; Infectious molecular clones |
| FACSDiva8 software | BD Biosciences | 659523 | FACS software |
| Fast SYBR Green Master Mix | ThermoFisher Scientific | 4385612 | qPCR reaction |
| FBS (Fetal Bovin Serum) | Atlanta BIO | S11050 | Cell Culture: Medium |
| Gateway System | Life Technologies | – | Plasmid Construction |
| Golden Gate System | in-house | – | Plasmid Construction |
| HEK 293FT | Invitrogen | R70007 | Cell Culture: Cells |
| Infusion Cloning System | Clonetech | 638920 | Plasmid Construction |
| JetPRIME reagent | Polyplus transfection | 114-15 | Transcfection reagent |
| Jurkat Cells | ATCC | TIB-152 | Cell Culture: Cells |
| L-Glutamine | Sigma-Aldrich | G7513-100ML | Cell Culture: Medium |
| Lipofectamine LTX with Plus Reagent | Thermo Fisher Scientific | 15338030 | Transfection reagent |
| LSR Fortessa flow cytometer (405, 488, and 561 nm lasers) | BD Biosciences | 649225 | Flow cytometer |
| MicroAmp Fast Optical 96-Well Reaction Plate (0.1 mL) | ThermoFisher Scientific | 4346907 | qPCR reaction |
| Neon Transfection System | Life Technologies | MPK10025 | Transfection reagent |
| Non-essential amino acids | HyClone | SH3023801 | Cell Culture: Medium |
| Opti-MEM I reduced serum medium | Life Technologies | 31985070 | Transfection medium |
| Penicillin/Streptomycin | Sigma-Aldrich | P4458-100ML | Cell Culture: Medium |
| QuantiTect Reverse Transcription Kit | Qiagen | 205313 | Rev Transcriptase kit |
| RNeasy Mini Kit | Qiagen | 74106 | RNA extraction kit |
| RPMI-1640 | ATCC | ATCC 302001 | Cell Culture: Medium |
| Shield | Clonetech | 632189 | Cell Culture: Drugs |
| SpheroTech RCP-30-5-A beads | Spherotech | RCP-30- 5A-2 | Compensation set up |
| StepOnePlus 7500 Fast machine | Applied Biosystems | 4351106 | qPCR reaction |
Referências
- Bernardo, D., Marucci, L., Menolascina, F., Siciliano, V. Predicting Synthetic Gene Networks. Synthetic Gene Networks: Methods and Protocols. 813, 57-81 (2012).
- Krams, R., et al. Mammalian synthetic biology: emerging medical applications. J. R. Soc. Interface. 12, (2015).
- MacDonald, J. T., Siciliano, V. Computational Sequence Design with R2oDNA Designer. Methods in molecular biology. 1651, 249-262 (2017).
- Zah, E., Lin, M. -. Y., Silva-Benedict, A., Jensen, M. C., Chen, Y. Y. T cells expressing CD19/CD20 bi-specific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunology Research. 4, 498-508 (2016).
- Wu, F., Bethke, J. H., Wang, M., You, L. Quantitative and synthetic biology approaches to combat bacterial pathogens. Current Opinion in Biomedical Engineering. 4, 116-126 (2017).
- Ye, H., et al. Pharmaceutically controlled designer circuit for the treatment of the metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 110, 141-146 (2013).
- Caliendo, F., Dukhinova, M., Siciliano, V. Engineered Cell-Based Therapeutics: Synthetic Biology Meets Immunology. Frontiers in Bioengineering and Biotechnology. 7, (2019).
- Thaker, M. N., Wright, G. D. Opportunities for synthetic biology in antibiotics: Expanding glycopeptide chemical diversity. ACS Synthetic Biology. 4, 195-206 (2015).
- Culler, S. J., Hoff, K. G., Smolke, C. D. Reprogramming cellular behavior with RNA controllers responsive to endogenous proteins. Science. 330, 1251-1255 (2010).
- Ausländer, S., et al. A general design strategy for protein-responsive riboswitches in mammalian cells. Nature Methods. 11, 1154-1160 (2014).
- Cella, F., Siciliano, V. Protein-based parts and devices that respond to intracellular and extracellular signals in mammalian cells. Current Opinion in Chemical Biology. 52, 47-53 (2019).
- Miki, K., et al. Efficient Detection and Purification of Cell Populations Using Synthetic MicroRNA Switches. Cell Stem Cell. 16, 699-711 (2015).
- Xie, Z., Wroblewska, L., Prochazka, L., Weiss, R., Benenson, Y. Multi-input RNAi-based logic circuit for identification of specific cancer cells. Science. , 1307-1311 (2011).
- Sun, J., et al. Engineered proteins with sensing and activating modules for automated reprogramming of cellular functions. Nature Communications. 8, 477 (2017).
- Shigeto, H., et al. Insulin sensor cells for the analysis of insulin secretion responses in single living pancreatic β cells. Analyst. 144, 3765-3772 (2019).
- Cella, F., Wroblewska, L., Weiss, R., Siciliano, V. Engineering protein-protein devices for multilayered regulation of mRNA translation using orthogonal proteases in mammalian cells. Nature Communications. 9, (2018).
- Siciliano, V., et al. MiRNAs confer phenotypic robustness to gene networks by suppressing biological noise. Nature communications. 4, 2364 (2013).
- Barnea, G., et al. The genetic design of signaling cascades to record receptor activation. Proceedings of the National Academy of Sciences of the United States of America. 105, 64-69 (2008).
- Schwarz, K. A., Daringer, N. M., Dolberg, T. B., Leonard, J. N. Rewiring human cellular input–output using modular extracellular sensors. Nature Chemical Biology. 13, 202-209 (2016).
- Wieland, M., Fussenegger, M. Engineering molecular circuits using synthetic biology in mammalian cells. Annual review of chemical and biomolecular engineering. 3, 209-234 (2012).
- Siciliano, V., et al. Engineering modular intracellular protein sensor-actuator devices. Nature Communications. 9, 1881 (2018).
- Lin, C. . HCV NS3-4A Serine Protease. , (2006).
- Romani, B., Engelbrecht, S., Glashoff, R. H. Functions of Tat: the versatile protein of human immunodeficiency virus type 1. Journal of General Virology. 91, 1-12 (2010).
- Basmaciogullari, S., Pizzato, M. The activity of Nef on HIV-1 infectivity. Frontiers in microbiology. 5, 232 (2014).
- Ross, C. A., Tabrizi, S. J. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet neurology. 10, 83-98 (2011).
- Pfeffer, C. M., Singh, A. T. K. Apoptosis: A Target for Anticancer Therapy. International journal of molecular sciences. 19, 448 (2018).
- Guzzo, C., et al. The CD8-derived chemokine XCL1/lymphotactin is a conformation-dependent, broad-spectrum inhibitor of HIV-1. PLoS pathogens. 9, 1003852 (2013).
- Marasco, W. A., LaVecchio, J., Winkler, A. Human anti-HIV-1 tat sFv intrabodies for gene therapy of advanced HIV-1-infection and AIDS. Journal of Immunological Methods. 231, 223-238 (1999).
- Gal-Tanamy, M., et al. HCV NS3 serine protease-neutralizing single-chain antibodies isolated by a novel genetic screen. Journal of molecular biology. 347, 991-1003 (2005).
- Southwell, A. L., et al. Intrabodies binding the proline-rich domains of mutant huntingtin increase its turnover and reduce neurotoxicity. The Journal of neuroscience the official journal of the Society for Neuroscience. 28, 9013-9020 (2008).
- Bouchet, J., et al. Inhibition of the Nef regulatory protein of HIV-1 by a single-domain antibody. Blood. 117, 3559-3568 (2011).
- Arold, S., et al. The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure. 5, 1361-1372 (1997).
- Beal, J. Signal-to-Noise Ratio Measures Efficacy of Biological Computing Devices and Circuits. Frontiers in Bioengineering and Biotechnology. 3, 93 (2015).
- Arold, S., et al. The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure. 5, 1361-1372 (1997).
- Tyshchuk, O., et al. Detection of a phosphorylated glycine-serine linker in an IgG-based fusion protein. mAbs. 9, 94-103 (2017).

.
