16.2:
緩衝液
A subscription to JoVE is required to view this content. Sign in or start your free trial.
JoVE Core
Chemistry
Buffers
Adding a small amount of acid or base to a solution can cause a significant decrease or increase in the pH. However, many chemical and biochemical processes need a stable pH to function. Buffers can prevent a drastic change in the pH of a solution when their buffering capacity is not exceeded. Buffers contain a weak acid and its conjugate base or a weak base and its conjugate acid. For example, human blood maintains its pH near 7.4 with a buffer composed of carbonic acid, a weak acid, and bicarbonate ions, its conjugate base. Conjugate acid-base pairs form buffers as they do not neutralize their conjugate acid or base. For example, acetic acid and acetate cannot react. However, if acetic acid, a weak acid, and ammonia, a weak base, are added together, they will react to form a salt—ammonium acetate. In a buffer, the weak acid neutralizes any added base by reacting with the hydroxide ions produced, whereas its conjugate base neutralizes any added acid by reacting with any hydronium ions. A similar mechanism works in the case of a weak base and its conjugate acid. Two beakers, X and Y, contain the same volume of different solutions, each with a pH of 7.2. The solution in beaker X is not buffered. In contrast, the solution in beaker Y contains an acetic acid-acetate buffer. If hydrochloric acid is added to beaker X, the pH of the solution suddenly drops due to the increased concentration of hydronium ions. In contrast, the solution in beaker Y shows a nearly constant pH when hydrochloric acid is added to it because one of the buffer components, acetate, reacts with hydrochloric acid to produce a chloride ion and acetic acid. Similarly, if sodium hydroxide is added to beaker X, the pH of the solution will suddenly increase due to the increased concentration of hydroxide ions. On the other hand, the solution in beaker Y shows a minimal change in pH when sodium hydroxide is added to it, as one of the buffer components, acetic acid, reacts with sodium hydroxide and produces sodium acetate and a water molecule. The buffer can prevent a drastic change in the pH of a solution as long as the concentration of the conjugate acid-base pair in a solution is higher than the added strong acid or base.
16.2:
緩衝液
弱酸とその共役塩基のペアを多量に含む溶液を緩衝液(バッファー)と呼びます。緩衝液は、少量の強酸または強塩基を加えてもpHが変化しにくい性質を持ちます。酢酸と酢酸ナトリウムの溶液は、弱酸とその塩からなる緩衝液の一例です。CH3COOH (aq) + CH3COONa (aq). 弱塩基とその塩からなる緩衝剤の例としては、アンモニアと塩化アンモニウムの水溶液があります。NH3 (aq) + NH4Cl (aq)。
緩衝液の働き
緩衝液の働きを説明するために、ほぼ同量の酢酸と酢酸ナトリウムの混合物を考えます。弱酸と共役塩基のペアが溶液中に存在することで、少量の強酸または強塩基を加えても中和することができます。例えば、この溶液に強塩基を加えると、ヒドロニウムイオンが中和され、酢酸のイオン化平衡が右にシフトして、減少したH3O+濃度が部分的に増加します。
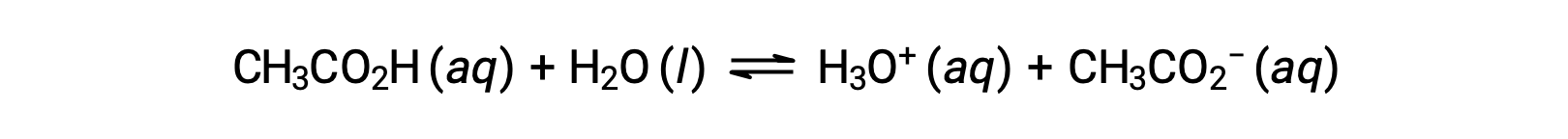
同様に、この緩衝液に強酸を加えると、酢酸イオンが中和され、上記のイオン化平衡が右にシフトし、[H3O+]が元の値に近づきます。Figure 1は、強酸や強塩基を加えたときの緩衝液の変化を図解したものです。溶液の緩衝作用は、新たに加えられた強酸や強塩基が、緩衝剤の共役ペアを構成する弱酸や弱塩基に変換されることによます。強酸と強塩基が完全に電離するのに比べて、弱酸と弱塩基はわずかに電離するだけです。そのため、溶液のpHは、緩衝剤を使用していない場合に比べて、急激に変化することはありません。
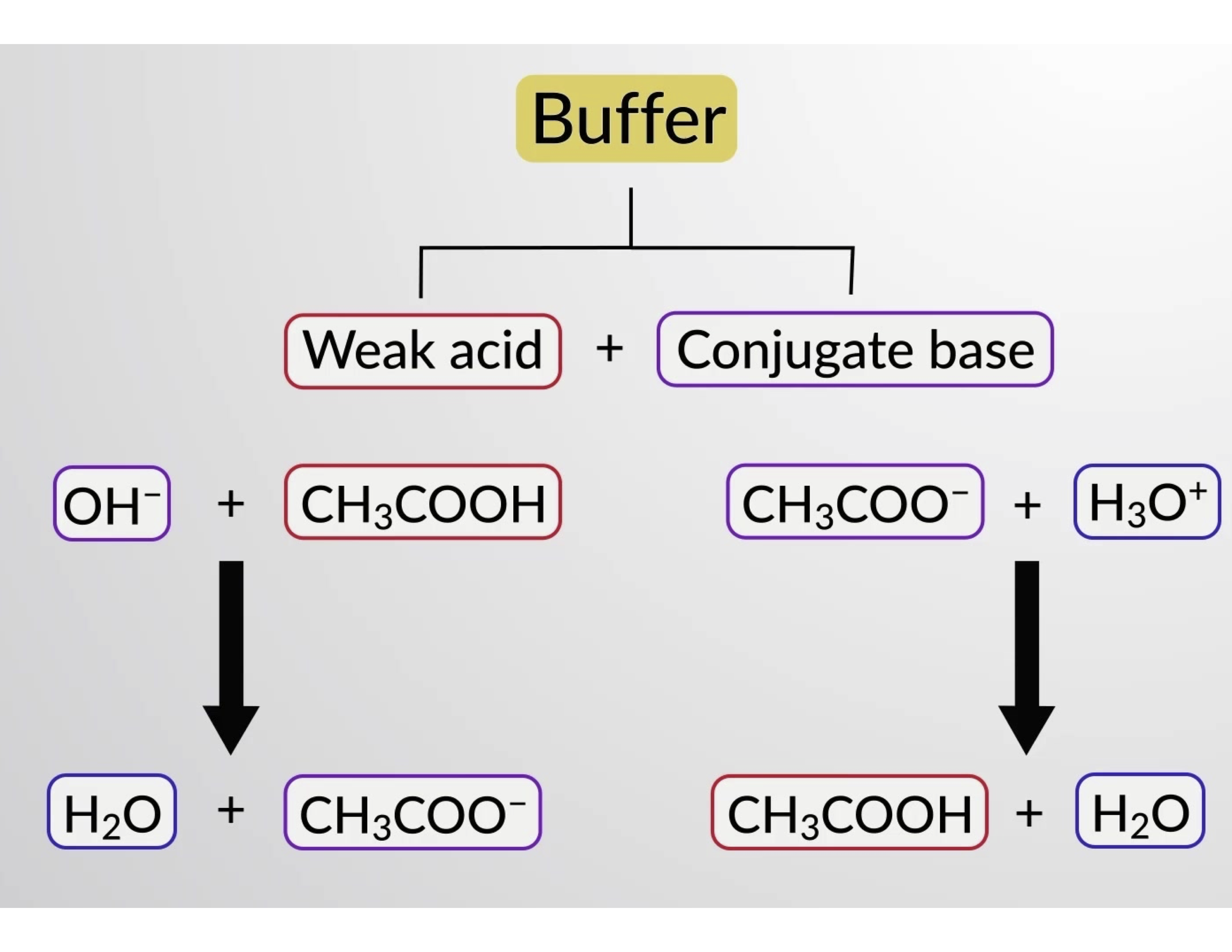
Figure 1. 酢酸と酢酸塩の混合液における緩衝作用
上記の文章は以下から引用しました。 Openstax, Chemistry 2e, Section 14.6: Buffers.
