The In Vitro Single Oocyte Reporter Assay: A Technique to Study Target mRNA Translation Regulation During In Vitro Oocyte Maturation
Abstract
Source: Costermans, N. G. J. et al., Defining the Program of Maternal mRNA Translation during In vitro Maturation using a Single Oocyte Reporter Assay. J. Vis. Exp. (2021).
In this video, we demonstrate that in the presence of a phosphodiester inhibitor, the oocyte cell cycle is arrested at prophase-I, during which transcribed mRNAs are stored in the absence of translation. After the release of the inhibitor, oocyte maturation resumes, and the cells enter metaphase. The signal of reporter mRNA fused with the target gene in metaphase-I indicates the activation of translational machinery and protein expression.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of media
- Add all components, as described in Table 1, to make the basic oocyte collection medium and oocyte maturation medium. For the basic collection medium, set the pH to 7.4. For both collection and maturation medium, add 3 mg/mL of bovine serum albumin (BSA) and 1 µM cilostamide on the day of use.
2. Preparation of mRNA encoding for Ypet-3' UTR and mCherry
- Obtain the 3′ UTR sequences of the mRNAs of interest.
NOTE: For this study, sequences were previously obtained from mouse oocyte cDNA. - Design primers to amplify the target 3' UTRs from oocyte cDNA and portions of the pcDNA 3.1 vector containing a Ypet coding sequence, V5-epitope tag, and a T7 promoter.
- Amplify using a high-fidelity DNA polymerase kit. Run the polymerase chain reaction (PCR) products on a gel to check if the fragments have the correct size, cut out the bands, and extract the DNA from the gel using a gel extraction kit according to the manufacturer's instructions.
- Fuse the PCR fragments to a vector using a PCR cloning kit.
NOTE: PCR fragments were incubated on ice for 4 h to facilitate a more efficient recombination process. This is contrary to the manufacturer's instructions, which recommend an incubation time of only 45 min. - Transfect PCR fragments into competent 5-α Escherichia coli bacteria.
- Add the mix and plasmid to the bacteria, and incubate on ice for 30 min.
- Heat shock the mix and plasmid by placing the mixture in a water bath at 42 °C for 45 s, and immediately cool on ice for 3 min.
- Add 500 µL of Super Optimal broth with Catabolite repression (SOC) medium and incubate for 1 h at 37 °C.
- Spin down at 7000 × g for 2 min, and remove most of the supernatant. Resuspend and plate on a Luria Broth (LB) agar plate with the appropriate selection antibiotic.
NOTE: Carbenicillin was used in this study. - Incubate overnight at 37 °C. Isolate the colonies by lightly pressing a pipette tip on one of the colonies and placing it in 3 mL of LB medium with 100 µg/mL of carbenicillin. Incubate for 12-24 h at 37 °C.
- Extract the DNA of the plasmids using the plasmid DNA isolation kit according to the manufacturer's instructions and confirm the sequences via DNA sequencing.
- For Ypet/3' UTR:
- Produce a linear PCR template for in vitro transcription. Use a forward primer upstream of the Ypet sequence and a reverse primer with 20 additional thymine residues. See Table 2 for the sequence of Ypet/IL-7 3' UTR and the sequences of the forward and reverse primers.
NOTE: These additional thymine residues will add oligo(A) to the linear mRNA after in vitro transcription. - In vitro transcribe the PCR product using a T7 transcription kit according to the manufacturer's instructions.
- Purify the resulting complementary RNA (cRNA) using a transcription clean-up kit according to the manufacturer's instructions.
- Elute the purified cRNA in RNAse-free water, measure concentration, and evaluate integrity by agarose electrophoresis. Store at −80 °C.
- Produce a linear PCR template for in vitro transcription. Use a forward primer upstream of the Ypet sequence and a reverse primer with 20 additional thymine residues. See Table 2 for the sequence of Ypet/IL-7 3' UTR and the sequences of the forward and reverse primers.
- For mCherry:
- Produce a linear PCR template for in vitro transcription by using a high-fidelity restriction enzyme; use the restriction enzyme mfEI-HF if replicating this example. Digest in a digestion buffer overnight at 37 °C.
- Run a gel to purify the sample, and extract the linear DNA using a gel extraction kit according to the manufacturer's instructions.
- In vitro transcribe the PCR product using a T7 transcription kit according to the manufacturer's instructions.
- Polyadenylate the cRNA (150-200 nucleotides) using a poly(A) tailing kit according to the manufacturer's instructions.
- Purify the resulting cRNA using a transcription clean-up kit according to the manufacturer's instructions.
- Elute the purified cRNA in RNAse-free water, measure mRNA concentrations, and evaluate the message integrity by gel electrophoresis. Store at −80 °C.
3. Experimental procedure
NOTE: A schematic overview of oocyte micro-injection and subsequent time-lapse microscopy is given in Figure 1.
- Day 1
- Intraperitoneally inject 21-day-old mice with 5 IU of pregnant mare's serum gonadotropin to promote follicle growth to the antral stage.
- Day 3
- Oocyte collection
- Carefully open the antral follicles by making a small cut in the follicle wall with a 26 G needle. Isolate intact cumulus-enclosed oocytes (COCs) with several layers of cumulus cells using a mouth-operated glass pipette.
- Use a smaller pipette (slightly larger than the diameter of the oocyte), and mechanically denude the COCs by repeated pipetting.
NOTE: Alternatively, perform micro-injection on intact COCs. - Using a larger pipette, aspirate the denuded oocytes and place them in a Petri dish with a maturation medium supplemented with 1 µM of the phosphodiesterase inhibitor, cilostamide, to prevent the resumption of meiotic maturation. Place the dish in the incubator for 2 h to let the oocytes recover from the stress induced by the isolation of the oocytes from the follicles.
- Oocyte micro-injection
- Prepare the injection needles by placing a 10 cm long borosilicate glass capillary tube in a mechanical puller. For optimal injection, bend the needle tip in a 45° angle using a heated filament.
- Prepare polystyrene dishes with 20 µL droplets of basic oocyte collection medium, and cover the droplets with light mineral oil.
- Prepare a reporter mix by adding 12.5 µg/µL Ypet-3' UTR and 12.5 µg/µL mCherry. Prepare a larger volume and make aliquots for future experiments to ensure similar reporter concentrations. Store these aliquots at -80 °C. Upon thawing, first centrifuge the aliquot for 2 min at 20,000 x g, and transfer to a new microcentrifuge tube.
NOTE: This centrifugation will prevent the injection needle from getting clogged by potential aggregates in the reporter mix. - Load the injection needle by capillarity with approximately 0.5 µL of reporter mix.
- Place the holding pipette and loaded injection needle into the holders, and position in the droplet of the oocyte collection medium. Aspirate some of the medium into the holding pipette.
- Open the injection needle by gently tapping it against the holding pipette.
- Place oocytes in a droplet of basic collection medium, and inject 5-10 pL of the reporter mix.
- Incubate the oocytes in the maturation medium with 1 µM cilostamide for 16 h to allow the mCherry signal to plateau.
- Prepare a Petri dish that will be used for time-lapse microscopy with at least two 20 µL droplets of maturation medium for each injected reporter: one droplet with 1 µM cilostamide for control prophase I-arrested oocytes and one droplet without cilostamide for maturing oocytes. Cover the droplets with light mineral oil and place in the incubator.
- Time-lapse microscopy
- After pre-incubation, remove the injected oocytes from the incubator, and wash them four times in maturation medium without cilostamide. Keep some oocytes in maturation medium with 1 µM cilostamide as a prophase I-arrested oocyte control group.
- Transfer the injected oocytes to their respective droplets on the previously prepared time-lapse microscope dish. Cluster the oocytes by using a closed glass pipette (a closed pipette can be prepared by holding the tip in a flame for a few seconds).
NOTE: Clustering the oocytes helps to prevent their movement during the recording. - Place the dish under the microscope equipped with a light-emitting diode illumination system and a motorized stage equipped with an environmental chamber maintained at 37 °C and 5% CO2. To replicate this study, use the following parameters: filter set: dichroic mirror YFP/CFP/mCherry 69008BS; YFP channel (Excitation (Ex): S500/20 × 49057; Emission (EM): D535/30 m 47281), mCherry channel (Ex: 580/25 × 49829; Em: 632/60 m).
- Enter the appropriate settings for the time-lapse experiment (see Table of Materials for the software used in this study): Click on Apps | Multi-Dimensional Acquisition. Select the first tab Main and select timelapse, multiple stage positions, and multiple wavelengths.
- Select the tab Saving to enter the location where the experiment should be saved.
- Select the tab Timelapse to enter the number of time points, duration, and time interval.
NOTE: The duration of the time-lapse experiment depends on the animal species studied, as the timing of oocyte meiotic maturation differs among species. In this experiment with mouse oocytes, oocytes were recorded every 15 min for 16 h. - Select the tab Stage. Switch on brightfield and locate the position of the oocytes by opening a new window by selecting Acquire | Acquire | Show Live. Once the oocytes are located, switch back to the Multi-Dimensional Acquisition window, and press + to set the location of the oocytes.
- Select the tab Wavelengths, and set 3 different wavelengths for brightfield (exposure 15 ms), YFP (exposure 150 ms for Ypet/IL7 3' UTR), and mCherry (exposure 75 ms for Ypet/IL7 3' UTR).
NOTE: Adjust the exposure for each Ypet/3' UTR reporter based on the level of reporter accumulation and the injected volume. Ensure that the YFP signal falls in the center of the range of detection at the start of the experiment to prevent underestimation or saturation in case of activation of translation. For mCherry, adjust the exposure for each batch of mCherry as the signal depends on the number of adenine nucleotides that were added during the polyadenylation procedure. Because of the variation in polyadenylation efficiency, use the same batch of mCherry in different experiments for a better comparison between experiments. - Start the time-lapse experiment by clicking on Acquire. See Figure 1 for an example of a time-lapse recording in a single oocyte.
- Analysis of Ypet-3' UTR translation
- For this analysis (see Table of Materials for the software used in this study), perform two region measurements for each oocyte: the oocyte itself-click on ellipse region and surround the oocyte-and a small region close to the oocyte to be used for background subtraction-click on rectangular region.
NOTE: Ensure that the oocytes do not move out of the selected region during the time-lapse recording. Pay special attention to the placement of the region measurement around polar body extrusion, as this causes movement in the oocyte and may distort the recording. - Export the region measurement data to a spreadsheet by clicking first on Open Log and then on Log Data to export the data analysis software.
- For each individual oocyte and for all measured time points, subtract the background region measurement from the oocyte region measurement. Do this for YFP and mCherry wavelengths separately.
- For this analysis (see Table of Materials for the software used in this study), perform two region measurements for each oocyte: the oocyte itself-click on ellipse region and surround the oocyte-and a small region close to the oocyte to be used for background subtraction-click on rectangular region.
- Oocyte collection
Table 1: Preparation of media. List of components that need to be added to prepare basic oocyte collection medium and oocyte maturation medium.
| Basic oocyte collection medium | |
| Component | For 500 mL |
| HEPES modified Minimum Essential Medium Eagle | 7.1 g |
| Sodium bicarbonate | 252 mg |
| Sodium pyruvate | 1.15 mL |
| Penicillin/Streptomycin 100x | 5 mL |
| Ultrapure distilled water (Invitrogen, 10977-015) | Up to 500 mL |
| Maturation medium | |
| Component | For 500 mL |
| MEM alpha 1x | Up to 500 mL |
| Sodium pyruvate | 1.15 mL |
| Penicillin/Streptomycin 100x | 5 mL |
Table 2: Example of reporter and primer sequences Sequence of YFP/IL7 3'UTR reporter and sequences of forward and reverse primers that were used to produce a linear PCR template for in vitro transcription.
| Sequence | |
| Ypet/Interleukin-7 3’ UTR | GAGAACCCACTGCTTACTGGCTTATCGAAATTAATACGACTCACTATAGGGAGACCCAAGCTGGCTAGTTAAGCTTGGTACCGAGCTCGGATCCACCGGTCGCCACCATGGTGAGCAAAGGCGAAGAGCTGTTCACC GGCGTGGTGCCCATCCTGGTGGAGCTGGACGGCGACGTGAACGGCCACAAGTTCAGCGTGAGCGGCGAGGGCGAGGGCGACGCCACCTACGGCAAGCTGACCCTGAAGCTGCTGTGCACCACCGGCAAGCTG CCCGTGCCCTGGCCCACCCTGGTGACCACCCTGGGCTACGGCGTGCAGTGCTTCGCCCGGTACCCCGACCACATGAAGCAGCACGACTTCTTCAAGAGCGCCATGCCCGAGGGCTACGTGCAGGAGCGGACCA TCTTCTTCAAGGACGACGGCAACTACAAGACCCGGGCCGAGGTGAAGTTCGAGGGCGACACCCTGGTGAACCGGATCGAGCTGAAGGGCATCGACTTCAAGGAGGACGGCAACATCCTGGGCCACAAGCTGGAG TACAACTACAACAGCCACAACGTGTACATCACCGCCGACAAGCAGAAGAACGGCATCAAGGCCAACTTCAAGATCCGGCACAACATCGAGGACGGCGGCGTGCAGCTGGCCGACCACTACCAGCAGAACACCCCCA TCGGCGACGGCCCCGTGCTGCTGCCCGACAACCACTACCTGAGCTACCAGAGCGCCCTGTTCAAGGACCCCAACGAGAAGCGGGACCACATGGTGCTGCTGGAGTTCCTGACCGCCGCCGGCATCACCGAGGGC ATGAACGAGCTCTATAAGAGATCTTTCGAAGGTAAGCCTATCCCTAACCCTCTCCTCGGTCTCGATTCTACGCGTACCGGTCATCATCACCATCACCATTGAACAGGACATGTAGTAACAACCTCCAAGAATCTACTGGTT CATATACTTGGAGAGGTTGAAACCCTTCCAGAAGTTCCTGGATGCCTCCTGCTCAAATAAGCCAAGCAGCTGAGAAATCTACAGTGAGGTATGAGATGATGGACACAGAAATGCAGCTGACTGCTGCCGTCAGCATATA CATATAAAGATATATCAACTATACAGATTTTTGTAATGCAATCATGTCAACTGCAATGCTTTTAAAACCGTTCCAAATGTTTCTAACACTACAAAGTCTACAAAAAGCAAGGCTATGAAGATTCAGAGTCACCACTGTTTTCTT AGCAAAATGATGGTATGGTTAAACATTCATTGGTGAACCACTGGGGGAGTGGAACTGTCCTGTTTTAGACTGGAGATACTGGAGGGCTCACGGTGATGGATAATGCTCTTGAAAACAAGAGTCTATCTTAAAGCAGCAG CAAAAAGAAGCTTAAGGCACTTAAGGCATCAACAAATGTAGTTAAATATGAATGTATAACACATAACTTCAGTAAAGAGCATAGCAGATATTTTTAAATAAAAGTATTTTTAAAGATAGAAATGCACTTATTCCAAAGATACTGA ACCTTAGTATTCAGTCGCTTTTGACACTTGTGTATAATAAAGCTTATATAACTGAATTTTCAATTTGAAAAGTATATTTTTAAAAGAATAATATATGCTAGACTTTTAATTAATGTATATGTTTAATTTTGGCATTCTGTCTGTCTCT CTGTCTCTCTCTCTCTCTCTCTCTCTCTCTACCTATCTATCTATATATATAATTTTCATATACTACCAATTGCGTACTTTGGATAGTGTCTCTTTTTAACCTAAATGACCTTTATTAACACTGTCAGGTTCCCTTACTCTCGAGAG TGTTCATTGCTGCACTGTCATTTGATCCCAGTTTTATTGAACACATATCCTTTAACACACTCACGTCCAGATTTAGCAGGAGACTAGGACCCTATAACTTTGTTAAGAGAGAAAACACTAATTTCTTGTTTTATAGTAGGGTC TTATTCGTATCTAAGGCAGGCTAGGATTGCAGACATGAGCCAATATGCTTAATTAGAAACATTCTTTTTATGTTAAACTCATGTCTTTTACAAGATGCCTACATATATCCTATGTATATGCCTGTTTAAATCCTTTTTTGTAAGGT CTGCTGTCTTCCTTCAGTTGTAATGGAAAGAAACACTATGTTGTAGAGGCCAAATTTCTGAAAGTGATAAGGGTTTGCTTGTACTGAATTCTCATTCTCCTTGCTTTTTCCAGCCACGTGAGCATCTAGCTATCTATACGCT GGATGTATTTGACCGATGCCTGCTCCACTGGCACATTGCATGTGTGGTAGCCATGCCTTCTTGCTTCTCCTTTTCCCCAACCCCTATAATGCTCTACTCAGTGGTACAGATAGCTGGGATTATCACAATTTTGAGAGAAAC ACCAATTGTTTAAAGTTTGTTTCATAATCACCATTTGCCCAGAAAACAGTTCTCTCAACTTGTTTGCAACATGTAATAATTTAAGAAACTCAATTTTGTTAATGGACTTTCGATAACTTCCTTAGATATCCCACATCTCCTACGT GTCAGTCCTTTGTCCTGAGGAACTGGTAAAATGGGTAAGCCCTTAGCTAGCGAACTGAAGGCATTCGCATGTGTAAGATAATCTCTATACCTGCAAGGCTGTCTGGATGGCTCCCTACCAATATTGAACAATATTCTGATT TTGGCAAAATAAAGGATAATATTTT |
| Forward primer | GAGAACCCACTGCTTAC |
| Reverse primer | TTTTTTTTTTTTTTTTTTTTAAAATATTATCCTTTATTTTGCCAAAATC |
Representative Results
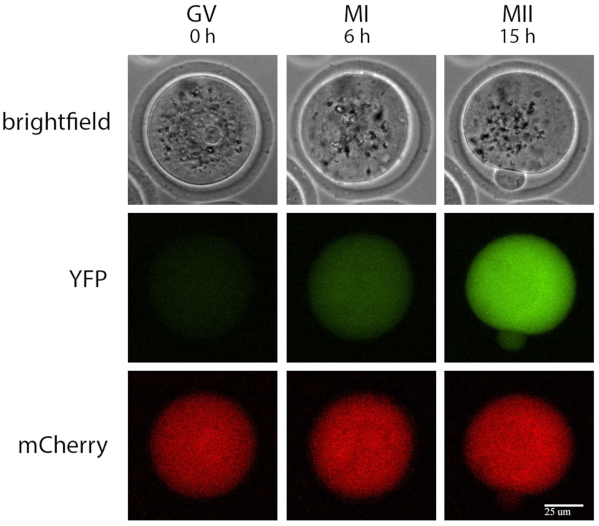
Figure 1: Example of a single oocyte time-lapse recording. Brightfield, YFP, and mCherry recordings of a single oocyte injected with mRNAs encoding Ypet/Interleukin-7 3' UTR and polyadenylated mCherry at prophase I, MI (6 h after cilostamide release), and MII (15 h after cilostamide release). Scale bar = 25 µm. Abbreviations: YFP = yellow fluorescent protein; GV = germinal vesicle; MI = metaphase I; MII = metaphase II.
Disclosures
The authors have nothing to disclose.
Materials
| Preparation of media | |||
| Bovine Serum Albumin Powder Bioxtra | Sigma-Aldrich | SIAL-A3311 | |
| Cilostamide | EMD Millipore | 231085 | |
| MEM alpha | Gibco | 12561-056 | |
| Minimum Essential Medium Eagle | Sigma-Aldrich | M2645 | |
| Penicillin-Streptomycin 100x Solution, Sterile Filtered | Genesee Scientific Corporation (GenClone) | 25-512 | |
| Sodium Bicarbonate | JT-Baker | 3506-1 | |
| Sodium Pyruvate | Gibco | 11360-070 | |
| Ultrapure distilled water | Invitrogen | 10977-015 | |
| Preparation of mRNA encoding YFP/3' UTR and mCherry | |||
| Agarose | Apex Biomedical | 20-102QD | |
| Carbenicillin disodium salt | Sigma-Aldrich | C1389-1G | |
| Choo-Choo Cloning Kit | McLab | CCK-20 | |
| CutSmart Buffer (10x) | New England Biolabs | B7204 | |
| DNA loading dye (6x) | Thermo Scientific | R0611 | |
| dNTP Solution | New England Biolabs | N0447S | |
| DpnI | New England Biolabs | R0176 | |
| GeneRuler 1 kb DNA ladder | Thermo Fisher | SM1333 | |
| LB Agar Plates with 100 µg/mL Carbenicillin, Teknova | Teknova | L1010 | |
| LB Medium (Capsules) | MP Biomedicals | 3002-021 | |
| MEGAclear Transcription Clean-Up Kit | Life Technologies | AM1908 | |
| MfeI-HF restriction enzyme | New England Biolabs | R3589 | |
| mMESSAGE mMACHINE T7 Transcription Kit | Invitrogen | AM1344 | |
| Phusion High Fidelity DNA polymerase | New England Biolabs | M0530 | |
| Poly(A) Tailing kit | Invitrogen | AM1350 | |
| QIAprep Spin Miniprep Kit | Qiagen | 27106 | |
| QIAquick Gel Extraction Kit | Qiagen | 28704 | |
| S.O.C. medium | Thermo Fisher | 15544034 | |
| TAE buffer | Apex Biomedical | 20-193 | |
| Ultrapure Ethidium Bromide Solution | Life Technologies | 15585011 | |
| Oocyte collection | |||
| Aspirator tube assembly for calibrated micro-pipettes | Sigma-Aldrich | A5177-5EA | |
| Calibrated micro-pipettes | Drummond Scientific Company | 2-000-025 | |
| PMSG- 5000 | Mybiosource | MBS142665 | |
| PrecisionGlide Needle 26 G x 1/2 | BD | 305111 | |
| Syringe 1 ml | BD | 309659 | |
| Oocyte micro-injection | |||
| 35 mm Dish | No. 0 Coverslip | 20 mm Glass Diameter | Uncoated | MatTek | P35G-0-20-C | For time-lapse microscopy |
| Borosilicate glass with filament | Sutter Instrument | BF100-78-10 | |
| Oil for Embryo Culture | Irvine Scientific | 9305 | |
| Petri Dish | Falcon | 351006 | For micro-injection |
| Tissue Culture Dish | Falcon | 353001 | For oocyte incubation |
| VacuTip Holding Capillary | Eppendorf | 5195000036 | |
| Software | |||
| Biorender | BioRender | Preparation of Figure 1S | |
| MetaMorph, version 7.8.13.0 | Molecular Devices | For time-lapse microscopy, analysis of 3' UTR translation |

