Visualization of Larval Segmental Nerves in 3rd Instar Drosophila Larval Preparations
Summary
Drosophila melanogaster larvae provide an ideal model system to investigate the mechanisms of axonal transport within larval segmental nerves. Using this procedure, 3rd instar larvae carrying various mutations can be compared to wild type larvae.
Abstract
Drosophila melanogaster is emerging as a powerful model system for studying the development and function of the nervous system, particularly because of its convenient genetics and fully sequenced genome. Additionally, the larval nervous system is an ideal model system to study mechanisms of axonal transport as the larval segmental nerves contain bundles of axons with their cell bodies located within the brain and their nerve terminals ending along the length of the body. Here we describe the procedure for visualization of synaptic vesicle proteins within larval segmental nerves. If done correctly, all components of the nervous system, along with associated tissues such as muscles and NMJs, remain intact, undamaged, and ready to be visualized. 3rd instar larvae carrying various mutations are dissected, fixed, incubated with synaptic vesicle antibodies, visualized and compared to wild type larvae. This procedure can be adapted for several different synaptic or neuronal antibodies and changes in the distribution of a variety of proteins can be easily observed within larval segmental nerves.
Protocol
1. Preparation of Reagents
- Prepare 1x Dissection Buffer using 128 mM NaCl, 4mM CaCl2, 4mM MgCl2, 2 mM KCl, 5mM HEPES and 36mM Sucrose. PH the solution to 7.2 and filter sterilize.
- Prepare the fixative using 1:1 dilution of 16% formaldehyde and 1x Dissection Buffer.
- Prepare 1x PBT using 1x Phosphate Buffered Saline (PBS) at a PH of 7.2 and Triton X-100 in a 1:100 ratio.
- Prepare 5% Bovine Serum Albumin (BSA) with 0.01% Na Azide in 1x PBS.
2. Preparation for the Dissection
- Sylgard dishes are used for dissection. Prepare the dishes by pouring Sylgard 184 silicone elastomer base and curing agent mixture into a Petri dish. Allow the mixture to completely solidify and dry before using.
- Before the dissection, gather a clean pair of #5 forceps, a clean pair of straight blade vannas scissors, and pins.
3. Dissection of 3rd Instar Larvae
- Collect wandering 3rd instar larvae of a specific genotype onto a petri dish.
- Wash the larvae in deionized water to get rid of the food.
- Place a larva on the sylgard dish, with the dorsal side up. Pin the larva at its anterior and posterior ends.
- Place a drop of 1x Dissection Buffer on the pinned larva. Using fine scissors nip the larva on the dorsal midline near the posterior end. Using the scissors, cut the larval cuticle from the posterior end through to the anterior end.
- Place a drop of new 1x Dissection Buffer onto the dissected larva. Using a pin, pick one lateral edge of the cuticle near the middle of the larva and pin the cuticle. Using another pin, pin the second lateral edge of the cuticle as shown in the diagram. Two pins are used on each lateral side. See diagram below.
- Carefully remove the intestines, fat bodies, and trachea, leaving only the brain with the two optical lobes and ventral ganglia with the segmental nerves attached. The remainder of the procedure is done on the sylgard dish.
4. Fixation of the Dissected Larva
- Incubate the dissected larva in fix for 30 minutes, by replacing the fix every 15 minutes.
- After fixation, rinse the larva in 1x PBT for 30 minutes by replacing the buffer every 10 minutes. It is important to note that the larva should always be in a buffer.
5. Antibody Staining of the Dissected Larva
- Prepare the primary antibody by diluting it to the appropriate concentration in 5% BSA with Na Azide in 1x PBT. Optimal concentrations will differ for different antibodies.
- Replace the last 1x PBT rinse with the prepared primary antibody solution, and incubate in a humid chamber at 4°C overnight. The humid chamber is constructed by lining a plastic dish with kim-wipes soaked in water.
- The following day, rinse the dissected larva in 1x PBT for 30 minutes by replacing the buffer every 10 minutes.
- Prepare the secondary antibody by diluting it to the appropriate concentration in 5% BSA with Na Azide in 1x PBT, and incubate the larva with the secondary antibody solution at 25°C for an hour. Wrap the humid chamber in foil to prevent light, as secondary antibodies are light sensitive.
- After incubation, rinse the larva in 1x PBT for 30 minutes by replacing the buffer every 10 minutes.
6. Mounting and Visualization of the Dissected Larva
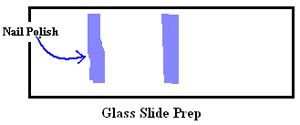
- Before mounting the larva on the slide, prepare the slide by spreading two vertical lines of nail polish in the middle, with a space enough for a cover slip to sit on (see diagram). Let the nail polish dry completely.
- Place a drop of mounting buffer in the space between the vertical nail polish lines on the slide (see diagram). The slide is now ready for mounting.

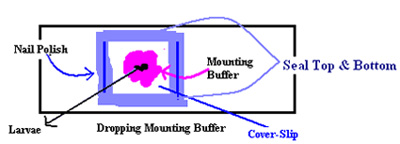
- After the last 1x PBT rinse, gently remove the lateral pins. Be careful to not tear the cuticle.
- Carefully remove the two remaining pins and pick up the larva with forceps and place the larva horizontally on the prepared slide. Make sure the larva is not flipped over and is oriented ventral side up.
- Gently place a cover slip on top and seal the edges with nail polish (see diagram). The larva is now ready for visualization under a fluorescent microscope.
7. Representative Results
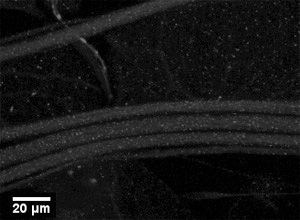
Figure 1: A representative image of larval segmental nerves from a wild type larva using antibodies against the synaptic vesicle protein cysteine string protein (CSP, Zinsmaier et al.1994 ). Note that the segmental nerves are smoothly stained. Bar=20μm
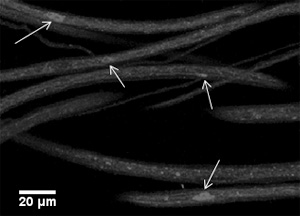
Figure 2: A representative image of larval segmental nerves from a motor protein mutant larva using antibodies against the synaptic vesicle protein cysteine string protein (CSP). Note that the segmental nerves show massive accumulations that stain brightly for CSP (arrows).
Discussion
The Drosophila larval segmental nerves are a powerful system to study mechanisms in axonal transport. If the 3rd instar larval dissection is performed correctly, all components of the CNS, PNS, and associated tissue, such as the muscles and NMJs, can be examined within a single larva. We have adapted this protocol from the one published by Hurd and Saxton (1996). This protocol can also be adapted to test other neuronal antibodies, but optimization of antibodies should be done. The primary and secondary antibody incubation times can be changed or a blocking step can be added. We have successfully used this protocol to investigate the role of the amyloid precursor protein 1 and Huntingtin 2 in axonal transport.
Disclosures
The authors have nothing to disclose.
Acknowledgements
SG is supported by funds from the State University of New York at Buffalo and from John R. Oishei Foundation.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Dumont #5 Forceps | Fine Science Tools, Inc. | 11252-20 | ||
| Minutien Pins, Stainless Steel | Fine Science Tools, Inc. | 26002-10 | ||
| Mcpherson-Vannas Straight Blade 8cm Scissors | Fisher Scientific | 50822236 | ||
| Vectashield, Mounting Medium | Vector Laboratories, Inc. | H-1000 | ||
| Sylgard 184 Silicone elastomer | Dow Corning Corporation | 4026148 | ||
| formaldehyde, 16% | Electron Microscopy Sciences | 15710 | ||
| dCSP3 | Developmental Studies Hybridoma Bank | |||
| Alexa Fluor 568 Goat Anti-Mouse IgG | Invitrogen | A-11004 |
References
- Gunawardena, S., Goldstein, L. Disruption of Axonal Transport and Neuronal Viability by Amyoid Precursor Protein Mutations in Drosophila. Neuron. 32 (2), 389-401 (2001).
- Gunawardena, S., Her, L. S., Brusch, R. G., Laymon, R. A., Niesman, I. R., Gordesky-Gold, B., Sintasath, L., Bonini, N. M., Goldstein, L. S. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron. 40 (1), 1-2 (2003).
- Hurd, D. D., Saxton, W. M. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 144 (3), 1075-1085 (1996).
- Zinsmaier, K. E., Eberle, K. K., Buchner, E., Walter, N., Benzer, S. Paralysis and early death in cysteine string protein mutants of Drosophila. Science. 263, 977-980 (1994).

