Isolating Cranial Neural Folds From a Chick Embryo for a Neural Crest Cell Culture
Abstract
Source: Jacques-Fricke, B. T., et al. Preparation and Morphological Analysis of Chick Cranial Neural Crest Cell Cultures. J. Vis. Exp. (2022).
This video illustrates a technique for isolating cranial neural folds from the midbrain region of chick embryos. It presents a detailed dissection process of the neural folds, their placement, and attachment on fibronectin-coated coverslips, followed by the observation of neural crest cell migration from the neural folds.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of solutions and materials
- Prepare Ringer's solution by mixing 123.3 mM NaCl, 1.53 mM CaCl2, 4.96 mM KCl, 0.809 mM Na2HPO4, and 0.147 mM KH2PO4 (see Table of Materials). Adjust pH to 7.4 and filter sterilize into 100 mL bottles. Store in a clean, dry location. Do not wash bottles with soap (treat as tissue culture glassware).
- Prepare 1 mg/mL stock solution fibronectin (FN) (see Table of Materials) by dissolving FN in sterile Ringer's solution and store in 100 µL aliquots at -80 °C (stable for at least 4 years).
- Prepare complete culture media by supplementing L15 media with 0.8% L-glutamine, 0.08% penicillin/streptomycin, 10% FBS, and 10% chick embryo extract. Create 3 mL aliquots and store at -20 °C or -80 °C.
NOTE: If cultures are incubated in a CO2 incubator, Dulbecco's Modified Eagle Medium F12 (DMEM/F12) needs to be substituted for L15, which is buffered for air. - Prepare filter paper support frames (approximately 1 cm x 1.5 cm rectangles of paper with two or three punched holes overlapping on the long axis).
- Punch holes along the edge of a large piece of filter paper using a standard three-hole punch, cut/trim the punched edge into a strip, and then cut the strip between the holes into rectangles ~ 1.5 cm in length. Autoclave filter papers before use (optional).
- Gather dissection tools, including fine forceps (#5), blunt forceps, dissection scissors, spring scissors, and sharpened tungsten needles.
2. Preparation of culture dishes
- Select neural fold culture dishes (see Table of Materials) appropriate for the downstream application.
- For cultures that will be fixed and stained, use glass coverslips placed into multi-well tissue culture dishes.
NOTE: Coverslips that match the well size will move less with fluid mixing, making explants less likely to shift and more likely to adhere to the coverslip rather than the dish bottom. - Select glass-bottom single chamber or divided chamber dishes (see Table of Materials) for live imaging with an inverted microscope.
- For live imaging on an upright or stereo microscope, use 6-, 12-, or 24-well optically appropriate tissue culture plates.
- Select plastic tissue culture dishes for the mass collection of migratory neural crest cells for high throughput analyses.
- For cultures that will be fixed and stained, use glass coverslips placed into multi-well tissue culture dishes.
- Add 10 U/mL of penicillin and 10 μg/mL of streptomycin to a 100 mL bottle of sterile Ringer's solution (for example, 100 μL of 10,000 U/mL penicillin and 10,000 μg/mL streptomycin, to make Ringer's P/S). Use Ringer's P/S within 1 week.
- Thaw an aliquot of 1 mg/mL of FN on ice. Dilute with Ringer's P/S to a concentration of 10-100 μg/mL of FN.
- Pipet enough FN solution to cover the bottom of the well or dish. For example, 100 μL per well in a 24-well plate, 500 μL for a 35 mm dish.
- Replace the lid and incubate dishes or plates with FN solution in a humidified incubator (or a covered tray with distilled water-soaked paper towels) at 38 °C (100 °F) for at least 1 h while dissecting neural folds.
3. Dissecting neural folds
- Transfer an embryo to a clean dish containing Ringer's P/S solution and rinse the embryo by holding the filter paper frame with forceps and gently swishing back and forth to clear away any yolk that obscures the view of the embryo. Exchange the Ringer's P/S or transfer to a fresh dish if it becomes cloudy.
- Position the embryo dorsal/frame side up under a dissecting microscope. Leaving the embryo on the paper frame to keep it stretched taut and held in place, remove the vitelline membrane using forceps to expose the neural folds.
NOTE: If the embryo falls off the paper frame, it can be laid out flat in the dish or pinned to a sylgard coated dish for dissection. - Using spring scissors or a sharpened tungsten needle, carefully excise the midbrain neural folds. Include tissue caudal to the expanding optic vesicles and rostral to the hindbrain, where rhombomere constrictions are just beginning to appear (the cardiac crescent is also a useful indicator, Figure 1B, C). Take care to excise the dorsal-most aspect of the neural fold with minimal contaminating neural tube and non-neural ectoderm (Figure 1C).
- Transfer the neural folds to a clean dish containing Ringer's P/S using a P20 pipettor or a sterile, glass Pasteur pipette rinsed with yolky Ringer's P/S (this blocks the plastic or glass to prevent the tissue from sticking). Store collected folds on ice while dissecting additional folds.
4. Plating neural folds
- Thaw an aliquot of complete culture media (section 1, step 3). Add 100 U/mL of penicillin and 100 µg/mL of streptomycin and filter sterilize. Keep prepared media at 37-38 °C while performing other steps.
- Remove culture dishes from the incubator (section 2, step 5). Using a pipettor or Pasteur pipette, remove the FN solution from coverslips, dishes, or wells. After rinsing the FN-coated substrate with Ringer's P/S, add an appropriate volume of complete culture media to the dishes or wells (500 µL for wells in a 24-well plate, 2 mL for a 35 mm dish or a six-well plate).
NOTE: Smaller volumes can preserve expensive reagents (e.g., as low as 200 µL for a 24-well plate) but ensure that the dish is sufficiently humidified to avoid evaporation. - Using a p20 or p200 pipettor, first, rinse the pipette tip with yolky Ringer's P/S to block the plastic and prevent the tissue from sticking. Then, transfer the isolated neural folds onto the FN coated coverslips, taking care to transfer as little Ringer's P/S as possible. Place the fold(s) toward the center of the FN-coated coverslip.
NOTE: One or a few neural folds can be plated on a 12 mm coverslip in a 19 mm well, up to 50 on a 35 mm plate. - After allowing the explants to settle for 10-15 min, place them into a humidified chamber at 38 °C (100 °F) by slowly and carefully carrying the culture dishes with plated neural folds. This will minimize the shifting of neural folds in the wells, ensuring they remain dispersed and adhere to any coverslips.
NOTE: When using L15 (not DMEM), explant culture dishes can also be incubated in an egg incubator using a covered tray with moistened paper towels. - Incubate neural fold cultures in the humidified incubator for the duration of active migration (about 16-20 h total, Figure 2).
Representative Results
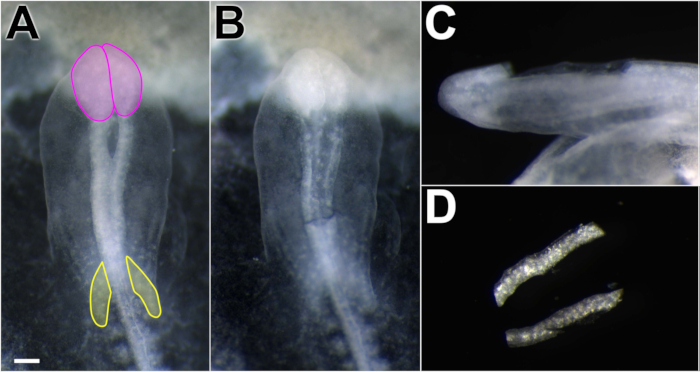
Figure 1. Dissection of chick dorsal neural folds. Working in Ringer's P/S, spring scissors were used to excise neural folds. (A) Embryo dorsal view, anterior toward the top of the figure. Neural folds appear more opaque than the surrounding tissue. Midbrain neural folds lie posterior to the optic lobes (pink) and anterior to the cardiac crescent (yellow). (B) Dorsal view of the embryo after neural folds were removed, showing excision boundaries. (C) Lateral view of the embryo with neural folds removed. The dissection technique removes the dorsal neural tube, avoiding ventral and non-neural tube structures. (D) Isolated neural folds in Ringer's P/S. Scale bar = 300 μm.
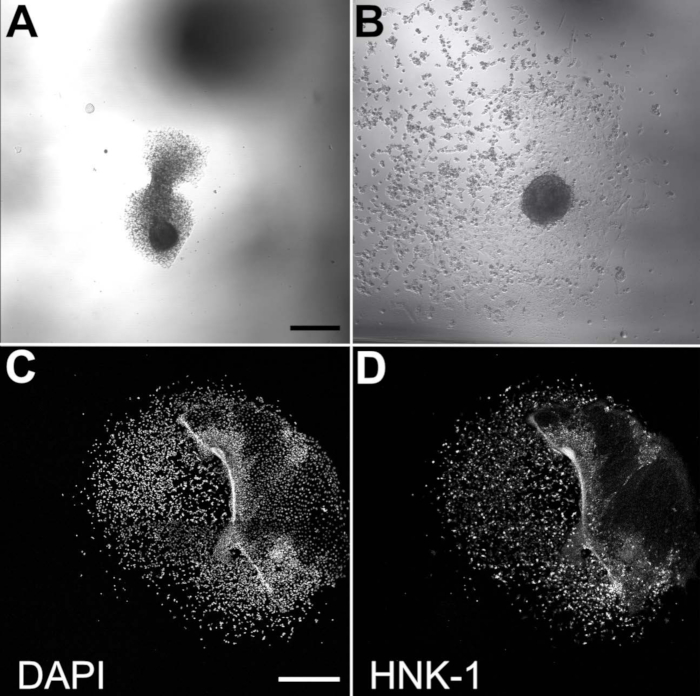
Figure 2. Migratory NCCs emerge from cultured neural folds. Brightfield images of plated neural folds after 3 h of incubation (A) and 20 h of incubation (B). Scale bar = 200 μm. (C,D) The neural fold is largely dispersed after 20 h of incubation but residually present on the right side of these images. Scale bar = 500 μm. Migratory NCCs are visible with DAPI (C) and HNK-1 staining (D). HNK-1 immunostaining confirms that cultured cells are migratory NCCs.
Disclosures
The authors have nothing to disclose.
Materials
| AxioObserver equipped with an LSM710 confocal scan head controlled by ZEN 3.0 SR software | Zeiss | Used alpha Plan-Apochromat 100x/1.46 Oil DIC M27 objective | |
| CaCl2 | Sigma-Aldrich | C3306 | |
| Chamber dishes (glass bottom, single or divided) | MatTek; Cell Vis | P35G-1.5-14-C (MatTek) X000NOJQGX (Cellvis) X000NOK1OJ (Cellvis) |
Single chamber 35 mm or 4 chamber 35 mm |
| Cover glass | Carolina Biological Supply Company | 633029, 633031, 633033, 633035, 633037 |
circles, 0.13–0.17 mm thickness, available in 12-25 mm diameter |
| DMEM/F12 | ThermoFisher Scientific | 11320033 | Alternative for L15 media |
| Egg incubator | Sportsman | 1502 | |
| FBS | Life Technologies | 10437-028 | |
| Fibronectin | Fisher Scientific | CB-40008A | |
| Filter paper | Whatman | grade 3MM chromatography | |
| Forceps (blunt) | Fisher Scientific; Thomas Scientific | 08-890 (Fisher);1141W97 (Thomas) | |
| Forceps (fine) | Fine Science Tools | 11252-20 | Dumont #5 |
| Pin holder | Fine Science Tools | 26016-12 | For tungsten needle (alternative for spring scissors) |
| KCl | Sigma-Aldrich | P3911 | |
| KH2PO4 | Sigma-Aldrich | P0662 | |
| L15 media | Invitrogen | 11415064 | |
| L-glutamine | Invitrogen | 25030 | |
| Na2HPO4 | Sigma-Aldrich | S9638 | |
| NaCl | Sigma-Aldrich | S9888 | |
| Penicillin/streptomycin | Life Technologies | 15140-148 | 10,000 Units/mL Penicillin; 10,000 mg/mL Streptomycin |
| Petri Dishes | VWR (or similar) | 60 mm, 100 mm | |
| Scissors (dissection) | Fine Science Tools | 14061-10 | |
| Spring Scissors | Fine Science Tools | 15000-08 | 2.5 mm cutting edge (alternative for tungsten needle) |
| Sylgard | Krayden | Sylgard 184 | |
| Syringe Filters | Sigma-Aldrich | SLGVM33RS | Millex-GV Syringe Filter Unit, 0.22 µm, PVDF, 33 mm, gamma sterilized |
| Tissue culture dishes | Sarstedt | 83-3900 | 35 mm culture dishes for bulk neural fold cultures |
| Tungsten wire | Variety of sources | 0.01" diameter for tungsten needle (alternative for spring scissors) |

