Mechanical Manipulation of Neurons to Control Axonal Development
Summary
Application and direct measurements of forces on neurons in the 2-1000 microdyne range are achieved with high precision using calibrated glass needles. This methodology can be used to control and measure several aspects of axonal development, including axonal initiation, axonal tension, velocity of axonal elongation, and force vectors.
Abstract
Cell manipulations and extension of neuronal axons can be accomplished with calibrated glass micro-fibers capable of measuring and applying forces in the 10-1000 μdyne range1,2. Force measurements are obtained through observation of the Hookean bending of the glass needles, which are calibrated by a direct and empirical method3. Equipment requirements and procedures for fabricating, calibrating, treating, and using the needles on cells are fully described. The force regimes previously used and different cell types to which these techniques have been applied demonstrate the flexibility of the methodology and are given as examples for future investigation4-6. The technical advantages are the continuous ‘visualization’ of the forces produced by the manipulations and the ability to directly intervene in a variety of cellular events. These include direct stimulation and regulation of axonal growth and retraction7; as well as detachment and mechanical measurements on any type of cultured cell8.
Protocol
1. Making glass needles.
- An adjustable micro-needle puller is used to fabricate needles with a tapered tip about 4 mm in length and that are closed solid beams. As opposed to a long flexible tip, this short 4 mm length limits vibrations of the needle tip during experiments. At the proximal region of the 4 mm fiber, the needle tapers rapidly from the diameter of the glass tubing to 15 μm within 1 mm, while the distal-most 1 mm of the fiber is 2.5 μm in diameter. We use R-6 glass capillary tube, OD 0.9 mm, ID 0.6 mm 8″ length and a BB-CH puller. If the investigator is interested in measuring other force ranges, needles with different spring properties (i.e. of a different length and or thickness) should be pulled. Each capillary tube pulls into four 2″ needles. Needles are stored in covered 160 mm Petri dishes with two modeling clay ‘snakes’ into which the needles are lightly pressed.
- The glass needles, as well as a wire needle to be described, are essentially beams used as bending springs of appropriate stiffness for the desired measurements of force. Note that the bending force of a beam scales as the cube of its length, regardless of composition. Thus, for any of the calibration steps described below, a needle/beam of insufficient stiffness can be made stiffer by a small amount of shortening (e.g. a 10% shortening produces a 28% increase in stiffness, i.e. 0.93).
2. Making and calibrating a wire needle
- Make a wire calibration needle by inserting a 0.001″ Chromel wire through the inside of a glass needle (made in section 1.1) with its tip broken off. Pull the wire out of the tip to a length of 26 mm and glue it in place with super glue. Bend the distal 1 mm of the wire into a hook.
- Make micro-weights from 1 meter of 0.003″ Constantan wire. To measure the wire, tape it to a meter stick to assist in getting it straight as possible. Weigh the 1 m of wire, accurately cut it to 1 cm pieces, and bend several pieces into Vs
- Calibrating the wire needles requires a dissecting microscope with a measuring reticle and a micromanipulator. The microscope is tilted 90° on its side using a universal boom stand, such that a downward deflection of the wire needle can be observed. Mount the wire needle of section 2.1 into a tool holder on a micromanipulator such that the hooked region is perpendicular to the optical axis of the microscope. Line up the hook of the wire needle with a reticle mark. Hang the 1 cm micro-weight on the hook and record the deflection. The bending constant (μdynes/μm) of the reference wire is calculated as the ratio of the micro-weight (note: 1 mg = 0.98 dynes) divided by the observed deflection (μm). To prepare the wire needle for the next step, cut off the hook at the weigh point with a scalpel. The presence of the hook is additional weight that bends the wire to a small degree. Thus it effects the initial position of the wire in the reticle before the weight is added. The measurement of the wire spring constant is derived from the wire’s deflection when the weight is added to the bend point (future tip of the wire). When the hook is cut off it does not change the spring constant of the wire.
3. Calibrating the intermediate glass needles
- Calibration of the intermediate glass needles requires the use of an inverted microscope with an ocular reticle. On one side of the microscope stage is a mechanical micromanipulator used to hold the wire needle of section 2 in the center of the optical field. On the other side is a three axis hydraulic micromanipulator (see equipment table) used to move the intermediate glass needle being calibrated. Using the micromanipulators, bring both needles together in the center of the optical field at an ~45°angle into a 160 mm x 30 mm Petri dish of water in a 10x field.
- A series of needles pulled with different settings on the pipette puller are first generated for evaluation. The goal is to make an intermediate glass needle with a bending spring constant of 20-30 μdynes/μm. Determination of the stiffness is done by measuring the ratio of the deflection of the intermediate needle as compared to the deflection of the wire needle. For example, with the wire and glass needles touching, the glass needle is moved to deflect the wire needle a distance of 5 marks on the reticle as seen through the eye pieces. Moving the glass needle sideways disengages the needles. The wire needle then returns to its original starting position, but the glass needle will now be in a new position. The load on the glass needle is then calculated from its new zero-force position minus the position of both needles at equal force load (both needles engaged). In this example, if both needles are deflected to mark 5 (from zero on the reticle), the wire needle will return to zero while the glass needle might spring to 25, if so, it bent 20 marks with the same force that flexed the wire needle 5 marks. Therefore, the ratio of bending between the two needles gives the calibration of the glass needle and it is ¼ as stiff as the wire needle. Because spring constant calculations in section 3.4 use ratios of distances they are independent of magnification or reticle division size. The only limiting consideration is that the deflections are small enough to be in the linear elastic range of the needles.
- Do a series of deflections at the positions of 5,10,15,3,7,12,17,5,10,15 marks on the reticle. If any deflections release prematurely repeat those. Between deflections keep the wire needle aligned at zero. For each of these deflections, note the amount deflected and the final zero-load position of the glass needle after release. This creates 10 pairs of data points. Other sequences may be used, but this series was designed to give early indications of internal consistency, linearity and hysteresis (results of deflections 5 + 10 = 15; 3 +7 = 10; the 5,10,15 deflections after release should be approximately the same at the beginning and end).
- Linear regression of the recorded deflections from section 3.3 gives a more accurate quantification of the bending constant of the intermediate glass needle than a single deflection ratio. It also provides an assessment of the linearity of the needle and quality of the calibration indicated by a higher r value and closeness of the intercept of the regression line to zero. To prepare the 10 data pairs from section 3.3 for the regression, the second number of the recorded pairs, the final zero-load position of the glass needle after release, must have the corresponding initial deflection subtracted from it, which is the first number of the pair. Once this operation is performed the set of new number pairs consist of the initial deflection and the corresponding difference calculated. Add 5 control pairs of 0,0 to these 10 experimental data pairs to anchor the linear regression at zero force for zero deflection. This is not absolutely required, but adds an extra degree of accuracy. Anchoring at 0,0 is based on the principle that the needle is not bent when force is not applied. With these 15 data pairs calculate a linear regression. The bending spring constant of the glass needle being calibrated is the wire needle’s known bending constant divided by the slope of this regression line.
4. Calibrating the working glass needle used to manipulate neurons
- Glass needles of sufficient compliance, 2-15 μdynes/μm, to be used experimentally on neurons are calibrated against the intermediate glass needle in an analogous manner as described in section 3. That is, candidate working needles deflect an intermediate needle of known stiffness placed in the position of the wire needle in section 3. Glass needles of less than 2 μdynes/μm may be slightly shortened to bring them into the appropriate stiffness range.
5. Needles are pretreated with attachment factors
- Needles to be treated are pressed into the side of a lump of clay held in a clamp set above a 10 ml beaker of attachment factor solution, such that only the tip of the needle is immersed in the solution. Immersing the capillary tube shaft is undesirable because it increases the accumulation of coating on the tip over time, which changes the stiffness and lessens the needle’s efficacy of attachment. A calibrated needle can only be used approximately 4 times and can be recalibrated for accuracy. The needles are dipped sequentially in 0.1% polylysine solution for 30 minutes and then concanavalin A (1 mg/ml in PBS) for 30 minutes. The polylysine can be used repeatedly for several weeks and is stored at -20°C. Make conA fresh weekly and store at 4°C. For other cell types the molecules presented to their surface by the needle may direct the course of the investigation.
6. Equipment set up
- The towing work station is set up on a vibration isolation table. It consists of an inverted microscope fitted with a bar along the left above the stage and a hydraulic manipulator attached to it, holding our counterbalanced custom extension, into which the double tool holder is inserted. Position the hydraulic micromanipulator along the bar, by putting a needle in its holder into the microscope’s light path, while the manipulator’s controls are centered in their ranges. At the time the needle is being treated with attachment factors, turn on the Ringcubator or other microscope stage heating system, so the stage is at thermal equilibrium at the beginning of the experiments.
7. Aligning the needles
- The objective is to get the two needles (the reference and working needle) in the microscope’s visual field arranged so their tips are about 50 μm apart, apart, with the reference above the culture dish, therefore somewhat out of focus when the towing needle is in focus on the dish. The two needles in their needle holders are mounted into a Leica double-tool holder, which is itself a small micromanipulator. Using the micromanipulation of the double-tool holder, the two tips are brought into parallel alignment. Insert the reference needle into a needle holder only a short distance, enough to allow the tightening collar to grip it and place this assembly in the right side of the double tool holder. Insert the treated working needle half its length into the needle holder and place it in the left side of the double tool holder. When the two needle tips are brought to the same forward position and lined up, the different lengths of the needles in their holders stagger the collars so they do not impede getting the tips very close together. Sections 7.2 through 7.4 are done without magnification. Sections 7.5-7.8 are done under microscopic observation.
- Pre-set the hydraulic micromanipulator so the vertical control is all the way up. Set the other controls to the center of their range, and also the forward/back screw of the tool holder.
- Bring the two needles tips together by using the side-to-side adjusting screw on the right side of the tool holder. Then push the needle holders themselves forward or back in the double tool holder as needed to bring the needle tips to the same forward position. Examine the pair from the side and use the right side up/down screw to bring the tips into the same plane. Rotate needle holders in the tool holder to close the gap if a needle angles in its needle holder.
- Swing the tool holder and place the tips into the light path above the surface of the media, angling them down steeply. Tighten the ball joint screw at the base before you move your hand away.
- Find your needle tips in the microscope’s visual field before you bring them too far down and break them or foul them with debris on the bottom of the culture dish. In a 60 mm dish, 15 ml of medium provides sufficient depth for a margin of safety above the dish bottom. Do an initial practice with an uncalibrated needle to get the feel of this step. Begin by focusing upward above the cells on bottom of the dish with a10x objective. Do this in an area of the dish away from light sensitive or exemplary experimental cells. Lower the needles into the media. Move the needles side to side looking for their shadow. If you don’t see them, lower them a little and move them forward in the dish, repeat the side to side pass. Find the tip, by moving back (or forward) and down.
- Turn the side-to-side screw to see which needle you have found. If it moves with the screw you are looking at the reference needle, if it doesn’t it’s the calibrated needle. For the calibrated needle, use the side to side screw to push the reference needle toward it. Move it forward and back looking for the reference’s shadow. If it is the reference needle you’ve found, screw it to the left while moving the hydraulic to the right looking for the calibrated needle.
- When both needles are located use the fine controls of the double tool holder to line them up. The steep angle of the needles coming over the edge of the dish causes the up/down control to also have a forward, lengthening component to the upward adjustment and a shortening with down. The left side, forward/back screw control has the opposite, pushing the tip forward is also down into the dish, pulling it back withdraws the needle while raising the tip’s position. Therefore, raising the reference and pushing forward the calibrated needle will lengthen both to make them even at the tips while also bringing them into the optimal relationship of heights above the dish. If the reference needle extends far beyond the working needle you can screw/push the working needle down and forward while swinging the reference down and shortening it until the two are even and the working needle is the lower. At times the screws range will not bring the needles together, so you may need to gently slide the holders themselves, make sure the ball joint screw is well tightened and use your fingers wedged against the edge of the tool holder to apply force to slide the shafts. You may also rotate the needle holders in the tool holder as before, while up in the media and observing the effect, seeing if they are getting closer together.
- Record the image of this final adjusted position, the separation of the needles without any force applied is the zero reference distance. Raise the needles above the specimen plane and ‘park’ them at the edge of the visual field.
- Debris on a needle may be removed by raising it through the meniscus. First move the reference needle away from the other needle to avoid the two tips latching onto each other. Multiple passes may be necessary.
8. Attaching to cells
- To this point, the needles have been observed directly through the microscope. Beginning with cell attachment, observations will be made both through the microscope and on the video screen. The left/right aspect of both is identical, but the up/down orientation of the image on the video screen is not the same as (gravitational) up and down on the microscope. To clarify the differences between the up/down directions as observed directly through the microscope and as observed on the video screen, the latter will be referred to as ‘up’ and ‘down,’ i.e. in quotes
- The process of towing is done by altering the position of the two needles from left to right (‘horizontal’) on the video screen. Consequently, the choice of experimental axon is made in part on the axon extending in the same ‘horizontal’ direction. The axon can deviate ‘downward’ from ‘horizontal’ by as much as 15°,as this will be compensated for by the ‘push up’ maneuver of section 8.5. In addition, the axon chosen for manipulation should be a cell body’s length or more, with an active growth cone. Axons extending to the right of the cell body are preferred due to the set up of the double tool holder with the working needle mounted to the left of the reference needle, such that applied tension during towing widens the gap between the two needles.
- A prime axon which extends to the left of the cell body can be towed without reorienting the double-tool holder set up by widening the gap between the needles and raising the reference needle a little. This allows the towing needle more space to flex toward the reference and pass beneath it if necessary. The towing will then advance to the left and the towing needle will bend toward the reference needle as tension increases. When analyzing the data this change in the relationship of tension to the reference distance is entered into the spreadsheet by subtracting the needle separation measurement from the zero reference distance instead of the zero distance from the measurement.
- Move the needles close to the growth cone of the axon, record the zero distance again, raise the needles, and air up the vibration isolation table.
- Simply putting the working needle in front of an advancing growth cone may produce spontaneous attachment as the growth cone advances. To pro-actively manipulate an attachment to the growth cone, place the towing needle down ‘below’ the growth cone and then lower the needle against the dish bottom. This will cause the needle to deflect ‘up’, bending and sliding along the dish into the growth cone, dislodging it and moving it ‘upward’. Press the needle against the dish bottom just enough so that the growth cone hooks around the needle tip, but not so far that it slips over the needle and slides backward. Wait 20 minutes to let a grip become established. With experience, the success rate can be as high as 90%, but is more typically in the 75% range. At first the success rate may be closer to 25%. The primary pitfall when beginning is to ‘over manipulate’ the growth cone. The initial interaction in the ‘push up step’ takes a few seconds. The growth cone should then be left alone to form a firm attachment. Then patience must be exercised during the following steps.
- When the growth cone is on the needle, put a slight tension on it by moving the needles to the right, and raise the needle a little. Compensate for the needle moving ‘downward’ by moving the needle forward a bit while raising. Raise in stages until you have the attached process extending perpendicular from the needle and above the surface of the dish. The perpendicular arrangement of axon extending from the needles once towing has begun is required for accurate force measurements, i.e. so that the sole applied force vector is along the axis of the axon. If the towed process angles away from the needle, a vector of the force is born along the axis of the needle rather than bending the needle and the total force is not measured. Getting away from the spot of the initial dish attachment improves the prospects of a lasting attachment to the needle. The tip may be above the surface of the dish (seen in little vibrations or a different plane of focus from the dish) or touching it with minimal force. Too much drag at the surface will skew the measurements. A needle sticking to the dish, or cell process reattaching to the substrate will lead to force loading if you keep trying to move it, which then suddenly ‘pops loose’ with possible loss of the attachment. To free the needle from such a sticking point, move the needle forward and back (‘up’ and ‘down’ in the plane of the screen) instead of adding more force (right).
9. Extending an axon or cell process
- Towing is accomplished by applying small increments of force by moving the needles away from the cell body. Forces are observed as an increasing gap between the needles. While gradually increasing the tension, carefully observe for any signs of detachment of the growth cone from the needle.
- If the growth cone detaches and isn’t rapidly retracting, decrease the tension slightly and wait, often the growth cone will ‘catch up’ and grab onto the tip again. If the axon comes off the needle catch it before it retracts by trapping it against the dish with the needle and repeating the ‘push up’ or a ‘pull down’ to put a little tension on it and wait about 10 min for re-attachment. After two failed attempts, we recommend using a different cell.
- Maintain the media level in the dish by adding water hourly to counterbalance evaporation, using a mark on the side of the dish to indicate the initial depth. With a long Pasteur pipette, slowly add water at the side of the dish away from the needles. Extension of a process often ‘stalls’ if the media becomes hyperosmotic. Evaporation could be diminished with an oil coating on the media surface, but this would have to be added after the needle is in the media. If the needle passes through oil, the growth cone is far less likely to attach to the needle.
- At the end of an experiment, before you lift the needles out of the dish, recheck the zero distance.
10. Data Analysis
- Read the positions of the needles along a horizontal axis on the screen, the position of the cell body and record times. The separation distance of the needles minus the unloaded zero distance multiplied by the bending constant for the needle equals the μdynes of force being applied. Calibrate the screen image distances using a stage micrometer.
- Very important for quality data is ensuring the reference distance (zero force) separation between the needles does not change during the experiment. Thermal changes will affect the equipment on the micron scale, i.e. don’t turn on the air conditioning during an experiment. Also if your microscope heating system has a lag time wait until you have thermal stability. The Ringcubator decreases the lag time and also isolates the thermal changes in the dish from the rest of the microscope and manipulation system diminishing thermal drift of the zero and changes in focus. Another force altering the needle’s unloaded separation is surface tension on the needle shafts. The load changes with the depth of liquid in the dish. Checking the reference distance by backing up the needles and temporarily seeing the no-force zero distance occasionally during an experiment improves the data quality, even if you are noting a change in the separation.
11. Representative Results:
The force-calibrated glass needles can easily be applied to any cellular region, usually the growth cone, as visualized directly by light microscopy. With appropriate treatment of the needle to obtain cellular attachment, mechanical tension can be experimentally applied to the cellular region of interest, or the forces produced by that region can be measured. A representative neuronal towing experiment is shown in Figure 1, where the towed axon has been doubled in length in two hours.
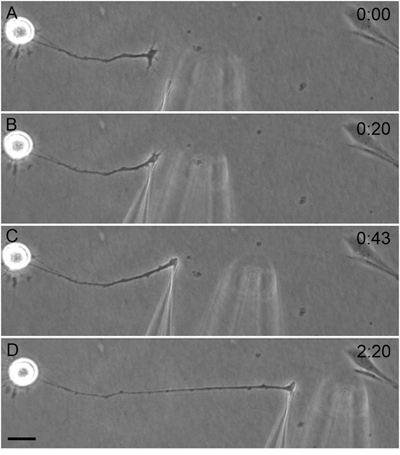
Figure 1. A cultured chicken dorsal root ganglion cell with the preferred orientation for towing is chosen. The initial zero reference distance between the needles is recorded before the experimental manipulation begins (A). The ‘push up’ maneuver is applied to the growth cone (B). Towing begins with increased force loading as indicated by the separation of the needles (C). Towing has extended the axon, the ‘horizontal’ and perpendicular alignments of the towed axon and working needle have facilitated good force measurements (D). Bar = 40 μm. Needle calibration = 6.9 μdyne/μm.
Discussion
Techniques to apply and measure cellular forces have a long history9. Our method was originally motivated by the work of Dennis Bray, who used glass needles similar to ours to ‘tow’ neurons at a constant rate using a motorized hydraulic device10. There are many alternative means of applying forces to cells which include: stepper motors11, magnetic beads12, microfabricated beams13 and fluid flows14. The latter are similar to our approach in that the cellular probe is ultimately calibrated against a bending beam of known stiffness and the cellular measurements depend on optical microscopic observations. In comparison to other approaches, the primary advantage of our method3 is the ability to simultaneously apply and measure forces in the μdyne range using equipment (microscopes and micromanipulators) typical of life science departments.
Using these methods, the forces exerted by neuronal growth cones as they advance have been measured15. The mechanical relationship of axonal elongation rate in response to tension has been determined to follow a Newtonian fluid-like stimulus-response relationship7. The relative sensitivity of axons in response to tension was shown to be greater for central brain neurons than for peripheral neurons of the chicken embryo2. Retraction of axons has also been investigated7. Neurons with no determined axons can have their axonal/dendritic polarity controlled by application of mechanical tension5. In fibroblasts expressing GFP-labeled actin or tubulin, we directly visualized the response of the cytoskeleton to simple attachment of the needle as well its response to changes of tension8. Cell attachments to each other or substrates have been tested1. The propulsive forces of motile cells could be examined and the pull of approaching filipodia could be measured16. The motions displayed by labeled mitochondria within towed axons have been used to model internal material transport (low velocity transport) and determine that neurons grow by intercalated mass addition along the length of the axonal shaft6,17.
For investigators simply interested in applying forces without measuring them, the use of the reference needle is not required18. In contrast, if force measurements are desired the reference needle is essential to generate accurate data. Overall, we estimate the error in the absolute force measurements to be in the range of 5% to 10% with the greatest source of error arising in the data reading process. The difficulty is in precisely identifying the location of the defocused reference needle and minimizing the effects of small back and forth movements (vibrations) of the needles. Without a reference needle, these sources of error would be even larger.
Experimental manipulations need not end with the towing session. Towed growth cones can be attached to other cells or reattached to the dish5. Using this manipulative opportunity, neuronal micro-circutry could intentionally be configured, allowing cross talk of different cell types to be examined.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We gratefully acknowledge the important contributions of Dr. Robert E. Buxbaum in the development of this methodology.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| R-6 cap. Tube | Drummond Scientific Co., Broomall, PA, USA | 9-000-3111 | R-6 glass OD 0.9mm, ID 0.6 mm, 8″ | |
| BB-CH puller | Mecanex S.A., Geneva, Switzerland | BB-CH puller | Use Mode 4 Alt by CP=100, PP=10, SP1=1000, SP2=1000 | |
| 0.001″ Chromel wire | Omega Engineering, Stamford, CT, USA | SPCH-001-50 | unsheathed, themocouple wire, 50ft spool now called Chromega | |
| 0.003″ Constatan wire | Omega Engineering, Stamford, CT, USA | SPCI-003-50 | unsheathed, themocouple wire, 50 ft spool | |
| fine forceps | Fine Science Tools, USA | 91150-20 | Dumont Inox #5 | |
| universal microscope boom stand | Nikon | 76135 or 90430 | most brands or types of boom stand will work for this use | |
| mechanical micromanipulator | Narishige | M-152 | three-axis direct-drive coarse micromanipulator | |
| hydraulic micromanipulator | Narishige | MO-203 | now available as MMO-203, three movable axis type | |
| needle holder | Leica Microsystems | 11520145 | set of 3 | |
| single instrument holder | Leica Microsystems | 11520142 | ||
| double instrument holder | Leica Microsystems | 11520143 | ||
| mechanical micromanipulator | Leica Microsystems | 39430001 | post mount,1 prob holder, RH Model 430001 | |
| joystick mech. micromanipulator | Leica Microsystems | 11520137 | ||
| Leica DM IRB | Leica Microsystems | inverted microscope | ||
| Vibraplane isolation table | Kinetic System, Boston, MA, USA | 1200 series | ours is model 1201-02-12 | |
| Ringcubator | self manufactured see reference 19 | reference 19, requires updated controller listed below | ||
| programable temperature controller | Instrumart.com | Fuji Electric PXR3 | replaces the retired PXV3 temperature controller | |
| Nikon Diaphot TMD | Nikon Instruments, Inc. | inverted microscope, circa 1980 | ||
| Nikon SMZ-10 binocular dissecting | Nikon Instruments, Inc. | other dissecting microscopes will work |
References
- Zheng, J., Buxbaum, R. E., Heidemann, S. R. Measurements of growth cone adhesion to culture surfaces by micromanipulation. J Cell Biol. 127, 2049-2060 (1994).
- Chada, S., Lamoureux, P., Buxbaum, R. E., Heidemann, S. R. Cytomechanics of neurite outgrowth from chick brain neurons. J Cell Sci. 110, 1179-1186 (1997).
- Heidemann, S. R., Lamoureux, P., Buxbaum, R. E., Haynes, L. W. . The Neuron in Tissue Culture. , 105-119 (1999).
- Lamoureux, P., Altun-Gultekin, Z. F., Lin, C., Wagner, J. A., Heidemann, S. R. Rac is required for growth cone function but not neurite assembly. J Cell Sci. 110, 635-641 (1997).
- Lamoureux, P., Ruthel, G., Buxbaum, R. E., Heidemann, S. R. Mechanical tension can specify axonal fate in hippocampal neurons. J Cell Biol. 159, 499-508 (2002).
- Lamoureux, P., Heidemann, S. R., Martzke, N. R., Miller, K. E. Growth and elongation within and along the axon. Dev Neurobiol. 70, 135-149 (2010).
- Dennerll, T. J., Lamoureux, P., Buxbaum, R. E., Heidemann, S. R. The cytomechanics of axonal elongation and retraction. J Cell Biol. 109, 3073-3083 (1989).
- Heidemann, S. R., Kaech, S., Buxbaum, R. E., Matus, A. Direct observations of the mechanical behaviors of the cytoskeleton in living fibroblasts. J Cell Biol. 145, 109-122 (1999).
- Yoneda, M. Force Exerted by a Single Cilium of Mytilus-Edulis .1. Journal of Experimental Biology. 37, (1960).
- Bray, D. Mechanical Tension Produced by Nerve-Cells in Tissue-Culture. Journal of Cell Science. 37, 391-410 (1979).
- Pfister, B. J., Iwata, A., Meaney, D. F., Smith, D. H. Extreme stretch growth of integrated axons. J Neurosci. 24, 7978-7983 (2004).
- Fass, J. N., Odde, D. J. Tensile force-dependent neurite elicitation via anti-beta1 integrin antibody-coated magnetic beads. Biophys J. 85, 623-636 (2003).
- Yang, S., Saif, M. T. A. Microfabricated Force Sensors and Their Applications in the Study of Cell Mechanical Response. Exp Mech. 49, 135-151 (2009).
- Bernal, R., Melo, F., Pullarkat, P. A. Drag Force as a Tool to Test the Active Mechanical Response of PC12 Neurites. Biophysical Journal. 98, 515-523 (2010).
- Lamoureux, P., Buxbaum, R. E., Heidemann, S. R. Direct evidence that growth cones pull. Nature. 340, 159-162 (1989).
- Heidemann, S. R., Lamoureux, P., Buxbaum, R. E. Growth cone behavior and production of traction force. J Cell Biol. 111, 1949-1957 (1990).
- O’Toole, M., Lamoureux, P., Miller, K. E. A physical model of axonal elongation: force, viscosity, and adhesions govern the mode of outgrowth. Biophys J. 94, 2610-2620 (2008).
- Bray, D. Axonal growth in response to experimentally applied mechanical tension. Dev Biol. 102, 379-389 (1984).
- Heidemann, S. R., Lamoureux, P., Ngo, K., Reynolds, M., Buxbaum, R. E. Open-dish incubator for live cell imaging with an inverted microscope. Biotechniques. 35, 708-708 (2003).

