Bridging the Bio-Electronic Interface with Biofabrication
Summary
This article describes a biofabrication approach: deposition of stimuli-responsive polysaccharides in the presence of biased electrodes to create biocompatible films which can be functionalized with cells or proteins. We demonstrate a bench-top strategy for the generation of the films as well as their basic uses for creating interactive biofunctionalized surfaces for lab-on-a-chip applications.
Abstract
Advancements in lab-on-a-chip technology promise to revolutionize both research and medicine through lower costs, better sensitivity, portability, and higher throughput. The incorporation of biological components onto biological microelectromechanical systems (bioMEMS) has shown great potential for achieving these goals. Microfabricated electronic chips allow for micrometer-scale features as well as an electrical connection for sensing and actuation. Functional biological components give the system the capacity for specific detection of analytes, enzymatic functions, and whole-cell capabilities. Standard microfabrication processes and bio-analytical techniques have been successfully utilized for decades in the computer and biological industries, respectively. Their combination and interfacing in a lab-on-a-chip environment, however, brings forth new challenges. There is a call for techniques that can build an interface between the electrode and biological component that is mild and is easy to fabricate and pattern.
Biofabrication, described here, is one such approach that has shown great promise for its easy-to-assemble incorporation of biological components with versatility in the on-chip functions that are enabled. Biofabrication uses biological materials and biological mechanisms (self-assembly, enzymatic assembly) for bottom-up hierarchical assembly. While our labs have demonstrated these concepts in many formats 1,2,3, here we demonstrate the assembly process based on electrodeposition followed by multiple applications of signal-based interactions. The assembly process consists of the electrodeposition of biocompatible stimuli-responsive polymer films on electrodes and their subsequent functionalization with biological components such as DNA, enzymes, or live cells 4,5. Electrodeposition takes advantage of the pH gradient created at the surface of a biased electrode from the electrolysis of water 6,7,. Chitosan and alginate are stimuli-responsive biological polymers that can be triggered to self-assemble into hydrogel films in response to imposed electrical signals 8. The thickness of these hydrogels is determined by the extent to which the pH gradient extends from the electrode. This can be modified using varying current densities and deposition times 6,7. This protocol will describe how chitosan films are deposited and functionalized by covalently attaching biological components to the abundant primary amine groups present on the film through either enzymatic or electrochemical methods 9,10. Alginate films and their entrapment of live cells will also be addressed 11. Finally, the utility of biofabrication is demonstrated through examples of signal-based interaction, including chemical-to-electrical, cell-to-cell, and also enzyme-to-cell signal transmission.
Both the electrodeposition and functionalization can be performed under near-physiological conditions without the need for reagents and thus spare labile biological components from harsh conditions. Additionally, both chitosan and alginate have long been used for biologically-relevant purposes 12,13. Overall, biofabrication, a rapid technique that can be simply performed on a benchtop, can be used for creating micron scale patterns of functional biological components on electrodes and can be used for a variety of lab-on-a-chip applications.
Protocol
1. Alginate Electrodeposition
- Connect a power supply to the custom fabricated electrodes via patch cords using alligator clips. An indium tin oxide (ITO) covered glass slide will act as the anode (working electrode) and platinum foil will serve as the cathode (counter-electrode). Position the electrodes so the ITO surface to be functionalized opposes the counter electrode and is positioned either vertically to be dipped into a solution or horizontally such that the deposition solution is contained on the surface.
- Prepare an alginate deposition solution by mixing 1% alginate and 0.5% CaCO3 (by weight) in distilled water and then autoclaving the solution. It is recommended to continuously stir the solution when not in use.
- Submerge both electrodes into the deposition solution. The alginate used may be fluorescently labeled with FluoroSpheres (Invitrogen), as per Cheng et al. 14, to allow for fluorescence imaging of the resulting film.
- Apply a constant current density (3 A/m2) for 2 minutes; voltage will shift within the range of 2-3 V.
- Disconnect the electrode and remove the non-deposited solution. Gently rinse the film with NaCl (0.145 M) to remove superfluous alginate.
- Incubate the film briefly (~1 min) in CaCl2 (0.1 M) to strengthen the gel. Rinse with NaCl (0.145 M) and incubate in a desired solution supplemented with CaCl2 (1 mM).
- Image using a fluorescence microscope (Figure 1B).
2. Codeposition of Communicating Cell Populations in Alginate
- A signal-sender cell culture (W3110 wt E. coli), grown in LB media, and a signal-receiver cell culture (MDAI2 + pCT6-lsrR–ampr + pET-dsRed–kanr), grown in LB media + 50 μg/mL each of kanamycin and ampicillin, must be grown up overnight and re-inoculated for growth to an optical density (at 600 nm) of 1. Adjust optical density of the receiver cell culture to 0.4-0.6 with LB before use.
- Prepare a deposition solution of 2% alginate and 1% CaCO3, and mix with each cell culture in a 1:1 ratio to a final concentration of 1% alginate, 0.5% CaCO3, with the cells diluted to a density of about half the culturing density.
- Use a glass slide patterned with two ITO electrodes contained by a polydimethylsiloxane (PDMS) well (prepared according to Sylgard instructions and cut to desired size) and a platinum counter electrode. Connect one ITO electrode to a power supply with the platinum as described in Procedure 1 (Alginate electrodeposition).
- Immerse the electrodes in a deposition solution containing the receiver cells. Set the power supply to a constant current at a density of 3 A/m2, where the surface area dimension is defined by the single electrode on which deposition will occur.
- Apply the current for 2 minutes to allow for codeposition of the cells in the alginate matrix.
- Rinse the film as described in Step 1.5.
- Switch the anodic connection to the second, adjacent, ITO electrode.
- Repeat the deposition procedure (Steps 2.4 – 2.7), but this time introducing the solution containing the sender cells.
- Incubate the 2-electrode chip containing codeposited cells and calcium-alginate overnight at 37 °C in phosphate buffered saline (PBS) supplemented with 10% LB media and 1 mM CaCl2.
- After incubation, image using a fluorescence microscope (Figure 2B).
3. Chitosan Electrodeposition
- Connect the power supply to the electrodes via alligator clips. A gold coated silicon chip will act as the cathode (working electrode) and a platinum foil will serve as the anode (counter-electrode). Position the gold electrode’s surface so that it opposes the counter electrode and both are positioned either vertically to be dipped into a solution or horizontally such that the deposition solution is contained on the surface.
- Prepare a chitosan solution by mixing chitosan flakes into water and slowly adding 2 M HCl to dissolve the polysaccharides (final pH 5.6), making sure to follow the procedure outlined by Meyer et al. 15.
- Place the electrodes into a chitosan solution (0.8 %), completely submerging the desired area for deposition. The chitosan used can be fluorescently labeled with 5-(and-6)-carboxyrhodamine 6G succinimidyl ester (Invitrogen), as per Wu et al. 8, to image the electrodeposited film by fluorescence microscopy.
- Apply a constant current density (4 A/m2) for 2 minutes. Voltage will shift within the range of 2-3 V. Calculate the current density as a function of the gold surface area of the working electrode exposed to the deposition solution.
- Rinse the electrode with DI water to remove superfluous chitosan. The chip can be stored in water or PBS (10 mM, pH 7.0).
- Image using a fluorescence microscope (Figure 3C).
4. Electrochemical Transduction with a Functionalized Chitosan Film
- Codeposit chitosan and glucose oxidase (GOx) from a solution (1% chitosan, 680 U/mL GOx, pH 5.6) at a current density of 4 A/m2 onto a patterned electrode according to Procedure 3 (Chitosan electrodeposition). A chitosan film entrapped in GOx will be generated.
- Attach the treated electrode to a three-electrode system as the working electrode, a platinum wire as the counter-electrode and Ag/AgCl as the reference electrode, as described in Figure 4A.
- Immerse the electrodes into a phosphate buffer solution (0.1 M, pH 7.0) containing NaCl (0.1 M).
- Electrochemically conjugate the protein to the chitosan film by applying a constant voltage (0.9 V) for 60s using chronoamperometry10.
- Place the chip in phosphate buffer (0.1 M, pH 7.0) and wash for 10 minutes on an orbital shaker to remove any unreacted NaCl and unconjugated GOx.
- Re-attach to the three-electrode system as described in step 4.2 and immerse into a solution of 5 mM glucose. Using cyclic voltammetry, sweep the potential in a positive direction to 0.7 V. Use a control film containing no glucose oxidase as a comparison for the amount of oxidation seen in the sweep (Figure 4B).
- Remove the electrodes from the glucose solution and rinse with phosphate buffer (0.1 M, pH 7.0) then place the electrodes into a 10 mL beaker containing 8 mL of phosphate buffer (0.1 M, pH 7.0). Bias the GOx-functionalized chip to 0.6 V to serve as the working electrode (Figure 4C).
- Add aliquots of glucose to the buffer (each aliquot increases the glucose concentration by 4 mM).
- Generate a standard curve between the steady state current and the glucose concentration for the GOx-functionalized chitosan film.
5. Protein Functionalization Using Enzymatic Assembly
- Use a glass slide patterned with an adjacent gold and ITO electrode contained within a PDMS well. Bias the gold electrode with a cathodic potential to electrodeposit chitosan as shown previously. Rinse film briefly in DI water and then PBS by pipette.
- Add a solution of 3 μM blue fluorescently labeled “AI-2 Synthase” 16 (using a DyLight labeling kit) + 100 U/mL Tyrosinase in PBS.
- Incubate for 1 h at room temperature, then rinse the film with PBS.
- Apply an anodic potential to the ITO electrode to codeposit an alginate deposition solution containing receiver cells (as prepared in steps 2.1-2.2). Follow steps 2.3-2.6 of Procedure 2 (Codeposition of cell populations in alginate).
- To generate the transmitted signal (AI-2) enzymatically, after rinsing the films add a solution of 500 μM S-adenosyl homocysteine (SAH) in PBS, supplemented with 10% LB media and 1mM CaCl2. Cover the electrodes to prevent evaporation of the solution and incubate overnight at 37 °C. This will allow for a receiver cell response by generating a red fluorescent protein (dsRed).
- Adjacent electrodes may be imaged with fluorescence microscopy by adjusting the filters to capture the blue fluorescence of the AI-2 Synthase and red fluorescence expressed by the codeposited receiver cells (Figure 5B).
6. Representative Results
Imposed electrical signals can create localized microenvironments (e.g., fields and gradients) near an electrode surface and these stimuli can trigger the self-assembly of polysaccharides such as alginate and chitosan to deposit as a hydrogel film on the electrode surface. Because this sol-gel transition occurs at the electrode surface, the resulting film is electroaddressed, with its geometry matching the electrode pattern (Figures 1B, 3C). Biocompatible films such as alginate and chitosan provide surfaces that can be functionalized with biological components. Using alginate, unique cell populations have been codeposited at separate addresses. Evidence of their electroaddressment is observed upon interaction between the sender and receiver cell population. The molecule autoinducer-2 (AI-2) diffuses from the sender cells and is taken up by the receiver cells, resulting in expression of the dsRed red fluorescent protein (Figure 2A). In Figure 2B, red fluorescence is observed only at the electrode where receivers are addressed.
The amine groups present on chitosan provide it with the pH responsiveness required for electrodeposition as well as a surface suitable for functionalization. We utilized these unique properties by electrochemically conjugating the biosensing enzyme glucose oxidase (GOx) to electrodeposited chitosan films. This enzyme then provides the ability for detection of glucose through an enzymatic reaction (Figure 4A) producing hydrogen peroxide which can then be electrochemically oxidized to produce an output current. In this way, a chemical signal can be transduced to electrical. Figure 4B shows that films in which GOx was electrochemically conjugated produce a strong anodic signal in the presence of glucose as opposed to those films containing no GOx. These results indicate GOx can be assembled onto a deposited chitosan film and will retain catalytic activity. Furthermore, Figure 4C shows a step-increase in anodic current produced in response to increasing glucose concentrations. The standard curve also present in Figure 4C shows that the step-increases proceeded in a near linear fashion dependent on the amount of glucose added. These results show that the enzyme also retains its sensitivity to increasing glucose concentrations upon conjugation to the chitosan film. The lower limit of detection was not studied here as it has been previously characterized for this system in the work of Meyer et. al.
We also have demonstrated the covalent immobilization of an enzyme of interest, engineered to contain a custom penta-tyrosine tag, to chitosan in an enzymatically-controlled manner. Specifically, this process is mediated by the enzyme tyrosinase. As depicted by the scheme in Figure 5A(upper), an enzyme, AI-2 Synthase includes a penta-tyrosine tag. Tyrosinase acts on the tyrosine tag, oxidizing the residues’ phenol groups to O-quinones, which then covalently bind to chitosan’s amines. Evidence of chitosan film functionalization with the AI-2 Synthase by tyrosinase assembly is observed in Figure 5B, where the AI-2 Synthase has been fluorescently labeled blue. Because AI-2 Synthase generates AI-2 from the substrate S-adenosyl homocysteine (SAH) in the same way as the sender cells, its proximity to codeposited receiver cells in the presence of SAH also causes the receiver cells to fluorescently respond by expressing dsRed (Figure 5A(lower)). Red fluorescence of the receiver cells (Figure 5B) again demonstrates interaction between addresses due to the diffusion of AI-2 from one to the other, and further indicates that enzymes immobilized to chitosan retain activity once covalently bound.
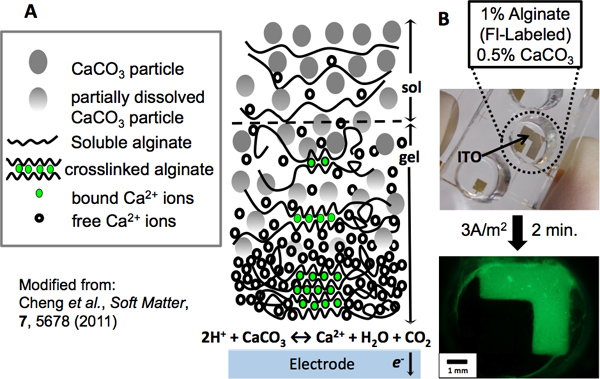
Figure 1. Alginate electrodeposition. (A) Mechanism of alginate electrodeposition: As an electrode is anodically biased, water electrolysis occurs at its surface, generating a localized low pH. Calcium carbonate particles react with the surplus of protons, releasing calcium cations as the particles dissolve. In the presence of alginate polymer chains, the ions become chelated in an “eggbox” network, forming a crosslinked hydrogel at the electrode. As the distance from the electrode increases, alginate has a greater tendency to remain in solution due to the reduced presence of calcium ions. (B) An L-shaped patterned ITO electrode was used to electrodeposit alginate. A PDMS well was fixed to the electrode to contain a green fluorescently-labeled alginate (1%) and CaCO3 (0.5%) deposition solution. After electrodepositing for 2 min. at a current density of 3A/m2, the electroaddressed alginate hydrogel was imaged by fluorescence microscopy.
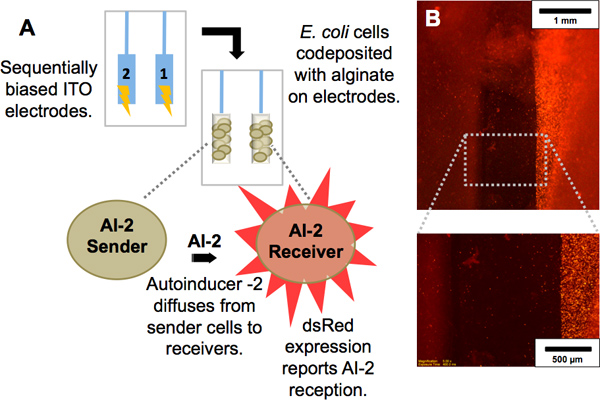
Figure 2. Codeposition of cell populations. (A) Scheme showing interaction between two E. coli strains: One population produces autoinducer-2 (AI-2), a signaling molecule, and is termed “AI-2 sender.” The other population, termed “AI-2 receiver,” is a reporter of AI-2; upon receipt of AI-2 by diffusion from the sender, it expresses the red fluorescent protein dsRed. (b) Red fluorescence image of electrode pair with the AI-2 sender population codeposited with alginate on the left electrode and AI-2 receiver population codeposited with alginate on right electrode. Magnified view demonstrates the dsRed expression of only the AI-2 receivers.
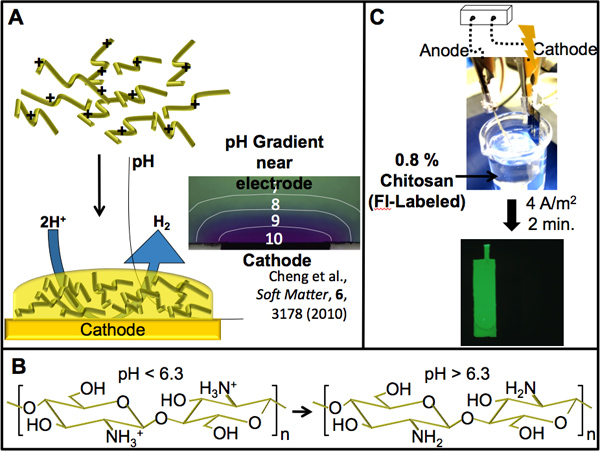
Figure 3. Chitosan electrodeposition. (A) Scheme showing the pH-dependent electrodeposition of chitosan. Water electrolysis at a cathodically biased electrode causes a localized high pH (shown by a localized color change of a pH indicator dye near the cathode in micrograph) which stimulates the sol-gel transition of chitosan in this region. (B) The amines present on chitosan give it pH-responsive properties. Above a pH of 6.3 (pKa of chitosan) the amines are deprotonated, facilitating a transition from its protonated soluble form to its insoluble gel form. (C) A patterned gold electrode was used to electrodeposit chitosan. The electrode, connected cathodically to the power supply, was immersed into a green fluorescently-labeled chitosan (0.8%) deposition solution. After electrodepositing for 2 min. at a current density of 4 A/m2, the electroaddressed chitosan film was imaged by fluorescence microscopy.
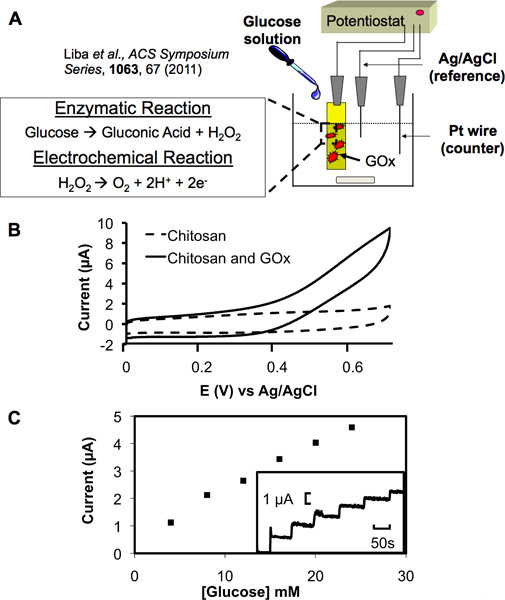
Figure 4. Electrochemical transduction with a functionalized chitosan film. (A) Schematic showing the set-up of a three electrode system. Functionalized chitosan film serves as the working electrode, a platinum wire as the counter electrode and Ag/AgCl as the reference electrode. Electrochemical transduction of glucose proceeds through the enzymatic and electrochemical reactions shown where produced hydrogen peroxide can be oxidized and detected at the working electrode. (B) Cyclic voltammagram (CV) at electrode with a chitosan film containing electrochemically conjugated glucose oxidase (GOx) shows a strong anodic signal in a 5 mM glucose solution. A film containing no GOx served as a control and displayed no signal in the same solution. (C) A standard curve between anodic current and the glucose concentration displays a near linear relationship (each aliquot increased the glucose concentration by 4 mM and also increased the current amplitude in the inset graph in a step-wise manner).
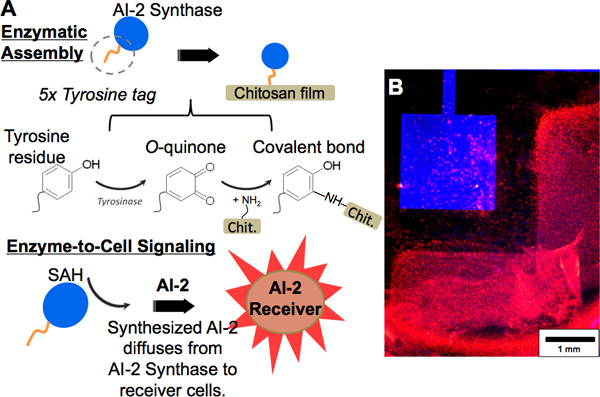
Figure 5. Protein functionalization using enzymatic assembly. (A, upper) Scheme showing tyrosine-tagged “AI-2 Synthase” being covalently tethered to a chitosan film by tyrosinase assembly. The tyrosine residues become oxidized to O-quinones by tyrosinase action and may react with amine groups on the chitosan film, forming a covalent bond. (A, lower) The AI-2 Synthase generates AI-2 from a substrate (SAH); the receiver cells report the generated AI-2 by dsRed fluorescence expression. (B) Fluorescence images showing a chitosan film on gold, functionalized with blue-labeled AI-2 Synthase. Adjacently, AI-2 receiver cells are codeposited with alginate on ITO. After addition of the enzymatic substrate to the well and incubation, the AI-2 receiver cells express dsRed.
Discussion
Our procedures demonstrate the electrodeposition and functionalization of biopolymer films, a process we term biofabrication. Through functionalization with cells and biomolecules we create biological surfaces capable of interacting with each other and the electrode address they are assembled upon. The first step, electrodeposition, takes place through the triggered self-assembly of biopolymers, alginate and chitosan in our studies, in response to an electrical signal. As stated earlier a pH gradient is generated which can be controlled by the current density and deposition time, providing additional control over the film dimensions and properties 6,17. We have found that a variety of current density and deposition time combinations can be used for the electrodes indicated in Table 1. While use of other electrodes is feasible, adjustments to the procedure would be necessary. Compared with other techniques of film formation the process of electrodeposition is simple, rapid and reagentless. There is no need for an extensive repertoire of expensive equipment and laborious preparations. Importantly, the process can withstand minor experimental deviations and can be easily started over if a problem occurs.
Chitosan is capable of responding to a high cathodic pH gradient due to important functional properties conferred to it by a high content of primary amines. At high pH (greater than its pKa of ~6.3) the amines are deprotonated and chitosan becomes insoluble, allowing for film formation. Following deposition, the films will remain attached to the electrode. However, the ability exists to delaminate them if desired. The films will remain stable as long as the pH of the solution does not drop below the pKa. Acidic solutions protonate the amines and the subsequent electrostatic repulsions swell the gel until it dissolves 18. That is, the assembly/disassembly process is reversible on demand and allows for removal of deposited films and reuse of electrodes. Conveniently, the pH range at which the sol-gel transition takes place is close to that in which most biological components function optimally. This makes the process ideal for the retention of functionality during assembly6.
Alginate film formation is facilitated by the anodic electrolysis of water as well as the presence of calcium carbonate 7. The localized low pH at the anode solubilizes the calcium carbonate leading to the release calcium cations. These ions are chelated by alginate, forming a crosslinked network on the electrode surface. Alginate films are notably reversible by competition for calcium ions from other chelating compounds such as citrate or EDTA, which can be used to dissolve the films, allowing for the re-use of the underlying electrodes. Thus, alginate films are relatively fragile when subjected to physiological conditions because calcium ions are easily scavenged from the gel matrix, weakening its structure and promoting film delamination or redissolution. To overcome this limitation, we have included an incubation step for the film in 1 M CaCl2 to strengthen the gel. Additionally, we recommend that the film’s incubation solution (cell media, etc.) be supplemented with CaCl2 at a concentration of 500 μM-3 mM.
The second major procedure is the functionalization of the deposited film with relevant biological components. This can be achieved in two ways, the first being electrochemical conjugation, a strategy that allows for rapid, reagentless assembly of proteins with exceptional spatial control 10. However, functionalization in this manner is limited by the diffusion of Cl– ions through the film to the electrode as well as the diffusion of HOCl, the generated reactive intermediate, back out into solution. The ability of electrochemically active molecules to pass through the film allows for the transduction of chemical and biological signals into easy-to-read electrical signals 15. We have shown tyrosinase-mediated coupling as a second strategy for enzyme functionalization to chitosan, demonstrated by covalently attaching AI-2 Synthase. This strategy allows the functionalization process to be controlled and selective – dependent on a specific reagent, tyrosinase, which acts discriminately on proteins containing a tyrosine tag 9.
We show the usefulness and biocompatibility of multi-address systems by replicating natural pathways on a chip. First we organized two cell populations (i.e. “senders” and “receivers”) at distinct addresses, and showed that they interacted across adjacent electrodes to deliver AI-2 and generate a fluorescence response. This concept has also been demonstrated by Cheng et al. in a microfluidic chip 14. We also mimicked the interaction, but instead used an enzyme to synthesize AI-2 for delivery. In this way, a synthetic intracellular pathway, AI-2 synthesis, was replicated through biofabrication and functioned much as it would in solution.
In both cases, assembly of multiple addresses presents the challenge of avoiding non-specific binding between addresses because each deposition solution must be introduced to the entire electrode array, even though electrodeposition is only intended at one address. Gentle yet thorough washing can remove the majority of residual solution from non-biased electrodes; the use of flow in microfluidic channels may further minimize non-specificity. Particularly for the adjacent biofabrication of chitosan and alginate addresses, we recommend depositing the chitosan film first, following this with biofunctionalization steps, and after this, electrodepositing alginate. Although we have not done so here, we have found that blocking the chitosan film with inert proteins (such as milk, BSA, etc.) greatly diminishes non-specific binding of unwanted molecules to chitosan’s aminated surface.
We have found utility in establishing patterned electrodes, often found in bioMEMS devices, as the “blueprints” for a complex arrangement of cells and biomolecules. The uses of electrodeposited chitosan in bioMEMS devices can go well beyond the examples mentioned here 19. Chitosan can be deposited on various microscale geometries – such as in microchannels and on non-planar surfaces 20,15. The films can also be modified with other polymers and a variety of proteins, DNA, nanoparticles, and redox-active molecules for novel properties 21,22,23. In bioMEMS devices, chitosan films have been used for drug delivery, redox and small molecule detection, biocatalysis, and cell studies 20,23,24,25. Similarly, alginate is widely used as a cell-entrapment matrix and has been explored for reversible fluidic containment of cell populations and in-film immunoanalysis 26,27,28. Composite films for tissue engineering applications have been fabricated using alginate electrodeposition, with components such as with hydroxyapatite for orthopedic implants 29.
In our demonstrations of biofabrication, we have shown both the interactions between biological components and across the bio-electronic interface to be equally applicable; this brings into reach the prospect of integrating all varieties of interactions for sophisticated performance in on-chip signal transmission. Accordingly, biofabrication may facilitate the fabrication of devices with reduced “minimum feature sizes” as a direct follow-on to rapid developments in microfabrication, as often motivated by consumer electronics. That is, next next-generation devices might in fact include labile biological components that offer nature’s exquisite assembly and recognition capabilities at even smaller length scales than man-made systems. We envision near-term applications in analytical instrumentation, environmental sensors, and even biocompatible implantable devices.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We acknowledge funding from DTRA for support of this manuscript and from ONR, DTRA, and NSF for partial support of underlying research.
Materials
| Name of the component | Company | Catalogue number |
| Power Supply | Keithley | SourceMeter 2400 |
| Three electrode potentiostat | CH Instruments | Potentiostat/Galvanostat 600D |
| RE-5B Ag/AgCl Reference Electrode with Flexible Connector | BASi | MF-2052 |
| Gold coated silicon wafer, 500um Si, 12nM Cr, 120nM Au, SiO2 for insulation | custom fabricated | |
| Indium Tin oxide coated glass slide, rectangular, 8-12 ohm resist | Sigma-Aldrich | 578274 |
| Platinum sheet/foil (0.002 in) | Surepure Chemetals | 1897 |
| Slim Line 2″ Alligator Clips | RadioShack | 270-346 |
| Multi-Stacking Banana Plug Patch Cord | TSElectronic | B-36-02 B-24-02 |
| SYLGARD 184 silicone elastomer kit | Dow Corning | NC9020938 From Fischer |
| Fluorescecence stereomicroscope | Olympus | MVX10 MacroView |
| cellSens Standard | Olympus | version 1.3 |
Table 1. Electrodeposition and fluorescence visualization equipment.
| Name of the reagent | Company | Catalogue number |
| Chitosan, medium molecular weight | Sigma-Aldrich | 448877 |
| Hydrochloric Acid, ARISTAR. ACS, NF, FCC Grade | VWR | BDH3030 |
| Sodium Hydroxide, Solution. 10.00N | VWR | VW3247 |
| Alginic acid, sodium salt | Sigma-Aldrich | 180947 |
| Multifex-MM Precipitated Calcium Carbonate, 70nm particles |
Speciality Minerals Inc. |
100-3630-3 |
Table 2. Chitosan and alginate solution reagents.
| Name of the reagent | Company | Catalogue number |
| Calcium chloride, dihydrate | J.T. Baker | 0504 |
| Sodium Chloride, Certified ACS crystalline |
Fischer Scientific |
S271 |
| Potassium Phosphate Monobasic, anhydrous | Sigma-Aldrich | P9791 |
| Potassium Phosphate Dibasic, anhydrous | Sigma- Aldrich | P3786 |
| Phosphate Buffered Saline | Sigma- Aldrich |
P4417 |
Table 3. Other solution components and buffer reagents.
| Name of the reagent | Company/Source | Catalogue number |
| Glucose oxidase from aspergillus niger | Sigma-Aldrich | G2133 |
| Tyrosinase from mushroom | Sigma-Aldrich | T3824 |
| LB broth, Miller (granulated) | Fischer Scientific | BP9723-2 |
| “AI2-Synthase” (HGLPT) | Lab stock 16 | |
| W3110 wildtype cells | Lab stock 30 | |
| MDAI2 + pCT6-lsrR–ampr + pET-dsRed–kanr cells | Lab stock 30 | |
| FluoroSpheres: 1μm diameter, Ex/Em: 505/515 | Invitrogen | F8765 |
| 5-(and-6)-carboxyrhodamine 6G succinimidyl ester, Ex/Em: 525/560 | Invitrogen | C-6157 |
| DyLight antibody labeling kit, 405 | Thermo Scientific | PI-53020 |
Table 4. Enzymes, cells, and other functionalization reagents.
References
- Luo, X. In situ generation of pH gradients in microfluidic devices for biofabrication of freestanding, semi-permeable chitosan membranes. Lab Chip. 10, 59-65 (2010).
- Javvaji, V., Baradwaj, A. G., Payne, G. F., Raghavan, S. R. Light-Activated Ionic Gelation of Common Biopolymers. Langmuir. 27, 12591-12596 (2011).
- Dowling, M. B., Javvaji, V., Payne, G. F., Raghavan, S. R. Vesicle capture on patterned surfaces coated with amphiphilic biopolymers. Soft Matter. 7, 1219-1226 (2011).
- Liu, Y. Biofabrication to build the biology-device interface. Biofabrication. 2, 022002-022002 (2010).
- Yang, X., Shi, X. -. W., Liu, Y., Bentley, W. E., Payne, G. F. Orthogonal Enzymatic Reactions for the Assembly of Proteins at Electrode Addresses. Langmuir. 25, 338-344 (2008).
- Cheng, Y. In situ quantitative visualization and characterization of chitosan electrodeposition with paired sidewall electrodes. Soft Matter. 6, 3177-3183 (2010).
- Cheng, Y. Mechanism of anodic electrodeposition of calcium alginate. Soft Matter. 7, 5677-5684 (2011).
- Wu, L. -. Q. Spatially Selective Deposition of a Reactive Polysaccharide Layer onto a Patterned Template. Langmuir. 19, 519-524 (2003).
- Wu, H. C. Biofabrication of antibodies and antigens via IgG-binding domain engineered with activatable pentatyrosine pro-tag. Biotechnol. bioeng. , 103-231 (2009).
- Shi, X. -. W. Reagentless Protein Assembly Triggered by Localized Electrical Signals. Adv. Mater. 21, 984-988 (2009).
- Shi, X. -. W. Electroaddressing of Cell Populations by Co-Deposition with Calcium Alginate Hydrogels. Adv. Funct. Mater. 19, 2074-2080 (2009).
- de Vos, P., Faas, M. M., Strand, B., Calafiore, R. Alginate based microcapsules for immunoisolation of islet cells. Biomaterials. 27, 5603-5617 (2006).
- Jayakumar, R., Prabaharan, M., Sudheesh Kumar, P. T., Nair, S. V., Tamura, H. Novel chitin and chitosan nanofibers and their biomedical applications. Biotechnol. Adv. 29, 322-337 (2011).
- Cheng, Y. Electroaddressing Functionalized Polysaccharides as Model Biofilms for Interrogating Cell Signaling. Adv. Funct. Mater. 22, 519-528 (2012).
- Meyer, W. L. Chitosan-coated wires: conferring electrical properties to chitosan fibers. Biomacromolecules. 10, 858-864 (2009).
- Fernandes, R., Roy, V., Wu, H. -. C., Bentley, W. E. Engineered biological nanofactories trigger quorum sensing response in targeted bacteria. Nat. Nanotechnol. 5, 213-217 (2010).
- Yi, H. Biofabrication with chitosan. Biomacromolecules. 6, 2881-2894 (2005).
- Liba Benjamin, D., Aranha India, V., Kim, E., Payne Gregory, F. ACS Symposium Series Ch. 4. Renewable and Sustainable Polymers. 1063, 61-71 (2011).
- Koev, S. T. Chitosan: an integrative biomaterial for lab-on-a-chip devices. Lab Chip. 10, 3026-3042 (2010).
- Luo, X. Programmable assembly of a metabolic pathway enzyme in a pre-packaged reusable bioMEMS device. Lab Chip. 8, 420-430 (2008).
- Spinks, G. M. A novel “dual mode” actuation in chitosan/polyaniline/carbon nanotube fibers. Sensor Actuat B-Chem. 121, 616-621 (2007).
- Yi, H. Patterned assembly of genetically modified viral nanotemplates via nucleic acid hybridization. Nano letters. 5, 1931-1936 (2005).
- Kim, E. Redox-cycling and H2O2 generation by fabricated catecholic films in the absence of enzymes. Biomacromolecules. 12, 880-888 (2011).
- Xie, Y., Xu, B., Gao, Y. Controlled transdermal delivery of model drug compounds by MEMS microneedle array. Nanomedicine. 1, 184-190 (2005).
- Odaci, D., Timur, S., Telefoncu, A. A microbial biosensor based on bacterial cells immobilized on chitosan matrix. Bioelectrochemistry. 75, 77-82 (2009).
- Selimoglu, S. M., Elibol, M. Alginate as an immobilization material for MAb production via encapsulated hybridoma cells. Crit Rev Biotechnol. 30, 145-159 (2010).
- Braschler, T., Johann, R., Heule, M., Metref, L., Renaud, P. Gentle cell trapping and release on a microfluidic chip by in situ alginate hydrogel formation. Lab Chip. 5, 553-559 (2005).
- Yang, X. In-Film Bioprocessing and Immunoanalysis with Electroaddressable Stimuli-Responsive Polysaccharides. Adv. Funct. Mater. 20, 1645-1652 (2010).
- Cheong, M., Zhitomirsky, I. Electrodeposition of alginic acid and composite films. Colloid Surface A. 328, 73-78 (2008).
- Tsao, C. Y., Hooshangi, S., Wu, H. C., Valdes, J. J., Bentley, W. E. Autonomous induction of recombinant proteins by minimally rewiring native quorum sensing regulon of E. coli. Metab. Eng. 12, 291-297 (2010).

