Intubation-mediated Intratracheal (IMIT) Instillation: A Noninvasive, Lung-specific Delivery System
Summary
Intubation-mediated intratracheal (IMIT) instillation of reagents is an excellent, noninvasive method for studying respiratory disease, as well as a method for instilling therapeutic reagents directly into the lung. It is a rapid and highly reproducible method which is suitable for preclinical testing.
Abstract
Respiratory disease studies typically involve the use of murine models as surrogate systems. However, there are significant physiologic differences between the murine and human respiratory systems, especially in their upper respiratory tracts (URT). In some models, these differences in the murine nasal cavity can have a significant impact on disease progression and presentation in the lower respiratory tract (LRT) when using intranasal instillation techniques, potentially limiting the usefulness of the mouse model to study these diseases. For these reasons, it would be advantageous to develop a technique to instill bacteria directly into the mouse lungs in order to study LRT disease in the absence of involvement of the URT. We have termed this lung specific delivery technique intubation-mediated intratracheal (IMIT) instillation. This noninvasive technique minimizes the potential for instillation into the bloodstream, which can occur during more invasive traditional surgical intratracheal infection approaches, and limits the possibility of incidental digestive tract delivery. IMIT is a two-step process in which mice are first intubated, with an intermediate step to ensure correct catheter placement into the trachea, followed by insertion of a blunt needle into the catheter to mediate direct delivery of bacteria into the lung. This approach facilitates a >98% efficacy of delivery into the lungs with excellent distribution of reagent throughout the lung. Thus, IMIT represents a novel approach to study LRT disease and therapeutic delivery directly into the lung, improving upon the ability to use mice as surrogates to study human respiratory disease. Furthermore, the accuracy and reproducibility of this delivery system also makes it amenable to Good Laboratory Practice Standards (GLPS), as well as delivery of a wide range of reagents which require high efficiency delivery to the lung.
Introduction
Mice have been used to model numerous human disease manifestations, including a myriad of respiratory diseases. Surrogate disease models are often unable to recapitulate all aspects of a modeled disease, typically due to important physiologic or immune differences in the two host models. Thus, a goal of improving model systems is to develop approaches that allow surrogates to more closely mirror a disease process or host response as observed in the original host system. There are several key physiologic differences between mice and humans in the mechanism by which they inspire air. Included in these differences are significant ratiometric differences in size between the URT and LRT. It has been estimated that mice possess >100 fold the URT surface area relative to humans, normalized against total lung capacity1,2. Thus, the nasal turbinates of the mouse allow for more extensive filtering of inspired air to facilitate a much greater rate of breathing, which may have a significant impact on studies of pneumonia if infection of the nasal cavity plays a significant role in disease progression.
Several different approaches have been employed to instill bacteria into the lungs of mice to study human-like respiratory disease. The most common of these approaches is intranasal inoculation, in which a liquid suspension is applied at one or both nares of a mouse. While relatively simple, caveats such as instillation volume and type of anesthesia used can impact the efficiency of instillation into the LRT via intranasal inoculation3-5. Specifically, Miller et al. have shown that intranasal instillation of Francisella tularensis in volumes less than 50 µl did not result in instillation of the bacteria into the LRT6. They further observed better LRT instillation when using inhaled isoflurane as opposed to injected ketamine/xylazine for anesthesia. However, our experience with Yersinia pestis intranasal inoculation indicates more consistent inoculation can be achieved using ketamine/xylazine as compared to isoflurane (MBL, unpublished data). These differences could be attributed to pathogen used or to variation in laboratory procedures, but importantly highlight the potential variability in this technique. Furthermore, lungs harvested shortly after intranasal instillation show that a relatively low percentage of the initial bacterial inoculum reaches the lung (in the case of Y. pestis, only 10% were recovered 1 hr after instillation7), suggesting that a large number of bacteria could be retained in the URT (or swallowed into the GI tract). In certain disease models, this significant deposition of bacteria on the URT mucosa may confound our understanding of the disease progression if the organism is capable of colonizing the murine nasal cavity in a manner inconsistent with the human disease. For example, using in vivo imaging, it has been observed that Burkholderia pseudomallei, which does not colonize the human URT, causes an overwhelming opportunistic infection of the murine nasal cavity when delivered by the intranasal instillation method8.
Other methods for instilling bacteria into the lungs of mice have also been employed in infectious disease research. However, compared to intranasal instillation these methods tend to require more technical expertise and/or expensive equipment without eliminating the potential for infection initiation at multiple sites (e.g., aerosol [URT and LRT]; transoral [digestive tract and LRT]; and surgical intratracheal [LRT and blood stream]). Given the potential complications that could be associated with secondary sites of infection, we sought to develop an intratracheal approach which bypasses the URT and delivers pathogen directly into the lungs of anesthetized mice, but also limits inadvertent inoculation into the blood stream or GI tract. Towards this end, intubation-mediated intratracheal (IMIT) instillation was developed as a nonsurgical procedure which guarantees LRT instillation of inoculum by including an intermediate step to verify proper catheter placement prior to instillation. This method is described using dye instillation to visually demonstrate broad distribution of the inoculum throughout the lung, and P. aeruginosa instillation to demonstrate the highly efficacious delivery (>98% of the inoculum) of this method to the lung. Importantly, while originally developed for bacterial delivery, IMIT also offers an effective tool for: i) instillation of various molecules for the study of other respiratory disease models, ii) lung-specific therapeutic delivery, and iii) basic lung function studies, including targeted siRNA delivery to the lung.
Protocol
NOTE: All of the procedures described here were reviewed and approved by the University of Louisville Institutional Biosafety Committee (protocol # 13-056) and Institutional Animal Care and Use Committee (protocol # 13-064).
1. Preparation of Dye
- Dilute 0.1% (w/v) Coomassie Brilliant Blue in PBS and filter sterilize using a 0.45 µM syringe filter.
2. Preparation of Pseudomonas aeruginosa Culture
- 15 hrs before instillation, inoculate 3 ml of broth culture with a single bacterial colony.
- Grow the culture 15 hr at 37 °C on a shaker (200 rpm).
- Centrifuge 1 ml of culture in a 1.5 ml microfuge tube 12,000 x g for 30 sec.
- Remove the medium and resuspend the pellet in 1ml of PBS.
- Dilute an aliquot of the bacterial stock suspension 1:10 in PBS and measure the OD600 of the diluted bacterial suspension to determine the bacterial concentration.
- Dilute the bacterial stock suspension in PBS to the desired concentration of bacterial inoculum, using a delivery volume of 50 µl for IMIT inoculation.
3. IMIT Instillation
- Place a group of mice into isoflurane anesthesia induction chamber and anesthetize using a 2 – 3% isoflurane/oxygen mixture.
- At the initial onset of sedation, scruff the mouse, holding the mouse upright, and administer 10 µl of a 2% lidocaine solution by gavage needle to the back of the throat and allow the solution to drain down to the epiglottis. Return the mouse to the anesthesia chamber.
- Allow a minimum of 5 min to permit the lidocaine to take full effect as a local anesthetic.
- When mice have achieved desired level of sedation (breathing rate of ~60 bpm), reduce the isoflurane to 2% to maintain sedation.
- Preload dye or bacterial inoculum into a 250 µl gas-tight precision syringe fit with a 22 G long blunt needle.
- First draw up 150 µl of air, measured by the Teflon plunger of the syringe. Next, draw up 50 µl of inoculum by advancing the Teflon plunger from the 150 µl mark to the 200 µl mark on the syringe body.
NOTE: When the sample is ejected into an intubated mouse, the 50 µl suspension will be delivered first, followed by a 150 µl air cushion which will distribute the inoculum throughout the lung.
- First draw up 150 µl of air, measured by the Teflon plunger of the syringe. Next, draw up 50 µl of inoculum by advancing the Teflon plunger from the 150 µl mark to the 200 µl mark on the syringe body.
- Remove one mouse from the induction chamber and lay supine on an intubation platform. Secure the mouse to the platform by hooking its incisors with an O-ring attached to Velcro strip, and securing the Velcro to the platform. Raise the mouse to a 45° incline.
- Using a micro cotton applicator, retract the tongue with a rolling motion, using the dominant hand.
- With the nondominant hand, use an operating otoscope fit with an intubation specula to both maintain tongue retraction and visualize the glottis.
- With the dominant hand, use a guide wire threaded through a 20 G catheter to intubate the mouse, seating the catheter to a 10 mm depth into the mouse trachea (catheter is fit with a silicon sleeve with 10 mm of exposed catheter). Remove the otoscope/specula.
- Confirm that the mouse has been correctly intubated by securing the catheter with the nondominant hand while briefly attaching a Luer connected length of 1/16” clear tubing containing a colored dye.
NOTE: The dye will rapidly migrate back and forth in response to breathing. - Do not proceed with subsequent steps if confirmation of intubation has not been established at this point. If the intubation attempted failed, reset the catheter and guide wire for one additional attempt at intubation.
NOTE: It is unadvisable to attempt more than two intubations of a mouse in one session without causing trauma to the mouse. - Continue to secure the catheter with the nondominant hand while inserting the precision syringe/blunt needle containing the liquid suspension/air cushion.
- Dispense the liquid/air directly into the lung in a single fluid motion and immediately remove the needle/catheter from the mouse.
- Return the mouse to a cage and allow recovery from anesthesia.
4. Characterization of IMT Delivery
- After IMIT instillation, euthanize the mouse by CO2 asphyxia at an appropriate time post-inoculation.
- Secure the euthanized mouse on a dissection board and soak the chest and abdomen with 70% EtOH using a squirt bottle.
- If evaluating distribution of an imaging agent throughout the lung, remove the lungs from the animal using sterile technique and display the lungs as appropriate for imaging.
NOTE: Lungs may be prepared for additional histological staining techniques through appropriate fixation or cryopreservation. - If evaluating the bacterial burden of infected lung tissue, remove the lungs from the animal using sterile technique. Place lungs into a sterile, preweighed 1 oz sample bag. Weigh and record the weight of the sample bag + lungs.
- Add 1 ml of sterile 1x PBS to each sample bag+ tissues. Reseal sample bag.
- Homogenize tissues by gentle rolling a 25 ml serological pipette over the sample bag + tissue.
- Generate serial dilutions of the lung homogenate in sterile PBS and plate on agar plate (LB, or as appropriate to the bacterial species being studied):
- Conduct a six fold serial dilution in a U-bottom 96 well plate by multichannel pipettor then plate triplicate samples by multichannel on the agar plate.
- Incubate agar plates overnight at 37 °C and enumerate colony forming units the next day.
Representative Results
To visualize the distribution of instilled material via the IMIT method, 50 µl of 0.1% Coomassie Brilliant Blue dye was instilled into the lungs of an anesthetized mouse. The mouse was immediately euthanized and the lungs were removed by sterile necropsy. Figure 1 shows that the dye was delivered to all lobes of the lung.
To determine the amount of bacteria delivered to the lungs via the IMIT method, three groups of mice (n = 3) were instilled with three different concentrations of P. aeruginosa (1.21 x 108, 1.21×107, and 1.21 x 106 colony forming units [CFU] per 50 µl). Immediately after IMIT instillation, mice were euthanized, lungs removed, and bacterial numbers were enumerated and compared to the inoculums (Figure 2). Delivery of the inoculum is highly efficient via this method, with >98% of the inoculum recovered from the lungs of instilled animals. Furthermore, IMIT instillation was highly reproducible regardless of the concentration of the inoculum (R2 = 0.9951).
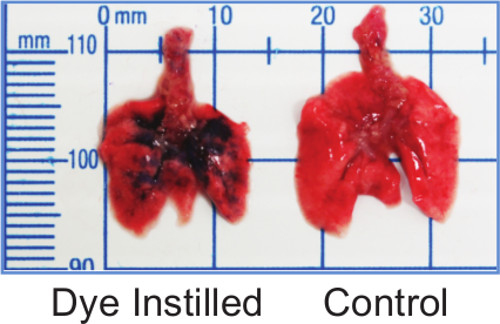
Figure 1: IMIT instillation distributes inoculum throughout the lungs. Lungs from mice instilled with 50 µl of 0.1% Coomassie Brilliant Blue show blue dye distributed in all lobes.
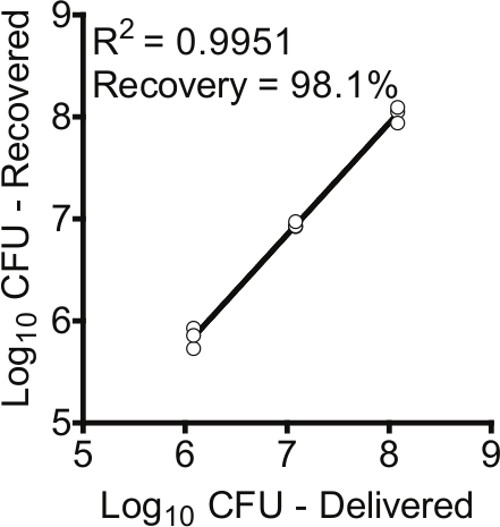
Figure 2: IMIT instillation of bacteria into lungs. Mice were instilled with P. aeruginosa and the number of bacteria instilled into the lungs (Log10 CFU – recovered) were compared to the estimated inoculum (Log10 CFU – delivered). Each circle represents the CFU/lung of an individual mouse (n = 3 for each bacterial dose).
Discussion
IMIT instillation offers key improvements to existing respiratory disease models in the ability to reproducibly instill reagents directly into the lung. It is a rapid approach which is ideally situated for a team of two researchers, one of which manages the logistics of anesthesia and caging, and the other who performs the IMIT technique. Large studies may be conducted using IMIT with an average time commitment of 2 – 3 min per mouse. Because the approach makes use of isoflurane as an anesthetic, mice recover rapidly from the anesthesia, reducing the husbandry time of monitoring animals through recovery.
The most technically challenging aspect of the IMIT method is the initial step of intubating mice. Individuals learning to perform IMIT are able to focus on this first step of catheter placement and ensuring that intubation has been achieved through the visual confirmation of dye movement. The benefit of the approach is that lung-specific instillation is guaranteed through use of the confirmation of intubation, which increases the confidence of both the new researcher as well as the expert attempting to intubate a difficult animal. The key elements of optimizing the likelihood of a successful intubation are: i) achieving a deep sedation to allow sufficient working time, ii) correct placement of the specula in the mouth to allow good visualization of the epiglottis, iii) good depth placement of the specula so that the tongue remains retracted throughout the procedure, and iv) use of the tilting platform to support the researcher’s hands so that the procedure is conducted relaxed and with a steady approach.
One of the limitations of the IMIT procedure is related to frequency of IMIT instillation events. Due to the potential trauma associated with a missed intubation, it is not recommended that more than two intubation attempts be conducted in a single session (up to two misses). IMIT has an excellent potential in its ability to be used to deliver therapeutics into the murine lung, however therapeutic regimens which make use of very frequent delivery of reagent into the lung may not be suitable for IMIT. It may be possible that IMIT could be used daily to deliver reagents into a murine lung without causing significant trauma, but only when conducted by a highly skilled researcher, as the majority of trauma associated with intubation is thought to be associated with a missed intubation event. Such high-frequency IMIT should be discussed with local veterinarians and IACUC.
An additional potential limitation of IMIT is the size of the mouse which is being intubated. The IMIT procedure described above was developed using mice of approximately 17 – 22 g, where a 20 G catheter was found to be a suitable size for the trachea of mice in this size range. Larger catheters have been successfully used in older mice; the initial development of IMIT made use of an 18 G catheter in BALB/c mice which are >20 g. Importantly, if alternate catheter sizes are used, blunt needles should be sourced which fit the lumen of the catheter and are trimmed to a length that extends just 1mm beyond the catheter tip. Intubation of mice smaller than 17 g may be possible but is not recommended due to the expertise required, and would require use of smaller catheters and specula than are described above.
We have used IMIT for the delivery of several respiratory pathogens in addition to P. aeruginosa, including B. pseudomallei9 and Klebsiella pneumoniae10. The IMIT model has made important advances to our studies of B. pseudomallei respiratory disease, having identified that intranasal inoculation causes an early, URT-related morbidity of mice rather than the systemic disease endpoint observed in human disease9. B. pseudomallei is a Tier 1 select agent of biodefense impact, and as such, respiratory disease models are being developed for aerosol exposure which models a potential biodefense related route of entry for weaponized pathogens. Because current aerosol models result in infection of both the URT and LRT, the same potential early morbidity phenotypes we have identified for the intranasal model of B. pseudomallei respiratory disease may apply to the aerosol model. A future adaptation of the IMIT model could be an intubation-mediated aerosol delivery (IMAD), in which mice are intubated for target aerosol delivery only into the lung. Mechanical ventilators are currently available to maintain isoflurane anesthesia, which could be adapted to deliver an aerosolized, rather than liquid based, pathogen challenge.
IMIT was developed initially as an approach to optimize the delivery of bacteria to the lung, but also has application for the delivery of other reagents into the mouse lung. As discussed above, intranasal delivery of compounds into mice results in a low efficiency, highly variable delivery of reagents into the target organ of the lung. Intranasal delivery of Positron Emission Tomography (PET) imaging reagents to the murine lung yielded a 40% delivery efficiency11, whereas we have demonstrated that IMIT offers an excellent alternative to other lung delivery approaches with its >98% delivery efficacy and multilobar distribution. This improvement in targeted delivery to the lung has the potential to increase the reproducibility of therapeutic delivery for treatment of pulmonary disease. IMIT could similarly offer benefits to studies of: i) the impact of environmental pulmonary irritants, ii) lung cancer phenotypic studies, iii) lung-specific siRNA knock-down.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors are grateful for the support from the Center for Predicative Medicine Animal Models (Carol Vanover, Ashley Biller and Jennifer Kraenzle) and Microbiology (Daniel Cramer and Julie Sotsky) Core Facilities. This work was supported by funding from the NIH (HHSN272201000033I to M.B.L and J.M.W.).
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| Rodent, Tilting WorkStand | Hallowell EMC | 000A3467 | Base should be detached when working in a BSC |
| Operating Otoscope Head | Welch Allyn | 21700 | |
| Otoscope 3.5 V Li Battery | Welch Allyn | 71900 | |
| Mouse Intubation Specula short, Autoclaveable | Hallowell EMC | 200A3589S | |
| Incisor Loops | Hallowell EMC | 210A3490A | |
| Cotton fine tip applicator | Puritan | 871-PC DBL | Used for tongue retraction |
| I.V. Catheter, 20G | Exel Int | 26741 | Optional: fit a silicon sleeve with 10mm exposed catheter surface |
| Gas tight syringe, 250ul | Hamilton | 81120 | Used for delivery of liquid inoculum by IMIT |
| Blunt Needle, 22G | Hamilton | 91022 | Trim to length to protrude 1mm from 20G catheter |
| Guide wire (Fiber optic wire, 0.5mm) | TheFiberOpticStore.com | FOF .50 | Cut to 6" length: used as guide wire for intubation |
| Tuberculin syringe, 1ml | Becton Dickinson | 309659 | Assemble with fiber optic wire as guide wire |
| Brilliant Blue R (Coomassie) | Sigma | B0149 | |
| Tygon tubing, 1/16" | Saint Gobain | ALC00002 | |
| Male luer 1/16" barb | Cole Parmer | 45503-22 | |
| Female luer 1/16" barb | Cole Parmer | 45500-00 | |
| Lidocaine, USP | Spectrum | LI102 | pH lidocaine into solution at 2%(w/v) pH7.0 |
| Sample bag, 1oz | Whirl-Pak | B01067 | |
| U-bottom 96 well plate, sterile | Greiner | 650161 |
References
- Reznik, G. K. Comparative anatomy, physiology, and function of the upper respiratory tract. Environ Health Perspect. 85, 171-176 (1990).
- Hoyt, R. F. J., Hawkins, J. V., St. Clair, M. B., Kennet, M. B., Fox, J. G., et al. Chapter 2. The Mouse in Biomedical Research, Volume 2, Second Edition: Diseases (American College of Laboratory Animal Medicine). 2, 23-90 (2007).
- Visweswaraiah, A., Novotny, L. A., Hjemdahl-Monsen, E. J., Bakaletz, L. O., Thanavala, Y. Tracking the tissue distribution of marker dye following intranasal delivery in mice and chinchillas: a multifactorial analysis of parameters affecting nasal retention. Vaccine. 20, 3209-3220 (2002).
- Eyles, J. E., Spiers, I. D., Williamson, E. D., Alpar, H. O. Tissue distribution of radioactivity following intranasal administration of radioactive microspheres. J Pharm Pharmacol. 53, 601-607 (2001).
- Southam, D. S., Dolovich, M., O’Byrne, P. M., Inman, M. D. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 282, 833-839 (2002).
- Miller, M. A., et al. Visualization of murine intranasal dosing efficiency using luminescent Francisella tularensis: effect of instillation volume and form of anesthesia. PLoS ONE. 7, (2012).
- Lathem, W. W., Crosby, S. D., Miller, V. L., Goldman, W. E. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proceedings of the National Academy of Sciences of the United States of America. 102, 17786-17791 (2005).
- Warawa, J. M., Long, D., Rosenke, R., Gardner, D., Gherardini, F. C. Bioluminescent diagnostic imaging to characterize altered respiratory tract colonization by the Burkholderia pseudomallei capsule mutant. Front Microbiol. 2, 133 (2011).
- Gutierrez, M., Pfeffer, T. L., Warawa, J. M. Type 3 Secretion System cluster 3 is a critical virulence determinant for lung-specific melioidosis. Submitted. , (2014).
- Fodah, R. A., et al. Correlation of Klebsiella pneumoniae comparative genetic analyses with virulence profiles in a murine respiratory disease model. PLoS ONE. In revision, (2014).
- Soto-Montenegro, M. L., et al. Assessment of airway distribution of transnasal solutions in mice by PET/CT imaging. Mol Imaging Biol. 11, 263-268 (2009).

