Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice
Summary
A protocol is provided to use an Open Field Maze to access general locomotor activity, anxiety and emotionality in a laboratory mouse model.
Abstract
Animal models have proven to be invaluable to researchers trying to answer questions regarding the mechanisms of behavior. The Open Field Maze is one of the most commonly used platforms to measure behaviors in animal models. It is a fast and relatively easy test that provides a variety of behavioral information ranging from general ambulatory ability to data regarding the emotionality of the subject animal. As it relates to rodent models, the procedure allows the study of different strains of mice or rats both laboratory bred and wild-captured. The technique also readily lends itself to the investigation of different pharmacological compounds for anxiolytic or anxiogenic effects. Here, a protocol for use of the open field maze to describe mouse behaviors is detailed and a simple analysis of general locomotor ability and anxiety-related emotional behaviors between two strains of C57BL/6 mice is performed. Briefly, using the described protocol we show Wild Type mice exhibited significantly less anxiety related behaviors than did age-matched Knock Out mice while both strains exhibited similar ambulatory ability.
Introduction
The Open Field Maze (OFM) was initially developed in 1934 as a test to measure emotionality in rodents1. It has attained the status of being one of the most widely used measures of behavior in animal psychology2. It provides an easy and fairly rapid assessment of well-defined behaviors requiring no training to the test subject and little to no specialized training for the human administering the test. These attributes have led to wide-spread use of the open field maze in research extended to other animal species such as calves, pigs, rabbits, primates, honeybees and lobsters3. Part of its popularity arises from the fact that the psychological and physiological concepts underlying the tests are generally straight-forward and well understood. For example, it has been postulated that evolutionary forces have selected for a common response in animals such that most species display anxiety-mediated fear or flight responses to specific stimuli. Rodents for example, show distinct aversions to large, brightly lit, open and unknown environments4. We can assume they have been phylogenetically conditioned to see these types of environments as dangerous. All of these features are incorporated in the open field maze and form the basis of its use in behavioral paradigm testing.
An open field maze consists of a wall-enclosed area that is of sufficient height to prevent the subject from escaping. Typical maze shapes are circular or square with an area large enough, based on the size of the subject tested, to elicit a feeling of openness in the center of the maze. A number of variables can be scored in the open field maze with most parameters involving differing types of motor activity2. Ambulation is the most common behavior studied but others such as latency or rearing can also be measured. Most often, rodent behavior is analyzed in a bare maze. However, the addition of objects, either one or many to the maze floor, adds the ability to see how the subject interacts with novel additional stimuli2. Relevant parameters when objects are presented are typically the number of approaches to an object or in some cases, preference or aversion for one object over another.
Many behavioral tests of anxiety are based on the subject animal’s body activity and locomotion5. Interpreting behavioral tests for emotionality while separating non-emotional confounding factors, such as motor activity, has been the subject of intense debate6,7. As the OFM was originally described, two measures of emotionality can be deduced, locomotor activity and fecal boli deposits or defecation1. However, these two measures have been shown in some studies to be unrelated supporting the conclusion that emotionality in rodents is multidimensional5. Regardless, discrepancies in the literature regarding these measures and emotionality or anxiety in mouse models may be attributed to differences in analysis criteria or differences in testing procedures. Studies have conclusively linked results from OFM analysis with other measures of anxiety when comparing mouse models8.
Protocol
NOTE: All procedures performed here were submitted to and approved by IACUC (Office of Research Compliance) and were conducted following NIH guidelines. Mice used in the behavioral testing paradigm were naive and not used for other tests. The C57BL/6 Wild Type and Knock Out mice used in this protocol have been described previously9 and the data presented here are from that manuscript.
1. Preparation of the Testing Room and Open Field Apparatus
- Use a multiple unit open field maze (OFM) consisting of four activity chambers was used for this analysis (Figure 1). Each chamber measured 50 cm (length) x 50 cm (width) x 38 cm (height) and was made from white high density and non-porous plastic.
- Texture the floors of the maze for traction during ambulation while maze walls were smooth. Maze quadrants were completely empty for the purpose of this test. In consideration of the rest of this protocol, a single quadrant of the maze described above will be utilized to demonstrate the OFM.
- Wipe the chamber with a 95% Ethanol prior to use and before subsequent tests to remove any scent clues left by the previous subject mouse.
- Allow the ethanol to evaporate completely prior to testing mice. This may take 5-10 min between each testing session.
- For this analysis, use the SMART Video Tracking software from PanLab/Harvard Apparatus to record and evaluate mouse movement.
NOTE: Any commercial video tracking camera and software may be used to track the test subject and evaluate results from the open field maze. It is very important for the end user to understand how to calibrate and run the software used for each individual analysis. Regardless of the video camera and tracking software used, best results are obtained when the camera and software are correctly calibrated according to the manufacturer’s instructions. - Perform the testing in a standard lit room capable of containing the maze apparatus and the computer required to run the software. Suspend the video camera above the maze either by attaching it to the ceiling or by using any elevated support system which allows the camera lens to see the entire maze area (Figure 2).
- As the human administrator of the test, be sure to have enough space in the room to be completely unobservable by the test subjects in the maze so as not to influence behavior of the mice.
2. Preparing the Software to Measure Activity
- Open the Video Tracking software.
- Once the software is opened, move the cursor to the “Single-subject tracking” option located under the “Data Acquisition” tab and single click to open this option.
- Choose the “Static Background” option located at the bottom of the screen.
- After choosing “Static Background” is chosen, it is necessary to use the software to take an image of the maze prior to addition of the test subjects. To do this, move the cursor to the “Photo” button located at the bottom of the screen and single click.
NOTE: The software will take a picture of the scenario without the test subject which will be subtracted from the image taken during the tracking process. This results in only the movement of the subject being analyzed by the software. - Confirm that the background image taken above is completely removed by the tracking software by moving the cursor to the “Test” button located at the bottom of the screen and clicking once. A solid white field will be shown if the background image is completely removed from the tracking image. If lighting conditions change or the maze is accidentally moved, you will see black “shadows” in this field indicating the two images do not perfectly coincide. To remedy this situation, simply repeat step 2.4 above.
- After confirming background settings, use the Timing option to configure the way time is controlled during acquisition. To do this, move the cursor to the “Configuration” tab and click once on the “Timings” heading. Use the newly opened window to enter experimental parameters.
- For this protocol, chose the “Programmed time” option as 10 min tracking period. Set the “Latency period” to 5 sec to allow the user time to place the mouse in the center of the maze and move away prior to initiation of tracking. Enter the “Acquisition time” of 10 min for the duration of the test. Set the “Stop control” set to “When programmed time (10 min) is over” which will automatically turn off the camera and the tracking function of the software.
- Move the cursor to the “Close” button after all timings have been set to close the window. You are now ready to begin the testing procedure.
3. Administration of the Open Field Test
NOTE: The software package used in this protocol allows the tracking of up to 16 individual mice at one time. For ease of completion and as mentioned above, the protocol discussed here is for a single mouse using a single quadrant of the OFM. For the equipment in use for this protocol, a maximum of 4 individual mice could be tracked using each quadrant of the maze. If utilizing a multi-enclosure maze, after placing the first subject mouse in its defined quadrant, place the remaining mice into their respective maze quadrant for tracking analysis. For the purposes of this protocol, further instruction will be specific to a single quadrant of the maze.
- Bring the mice in their home cages from their housing room into the testing room. Allow the mice to acclimate to the procedure room for a minimum of 30 min prior to starting the test.
- Remove a single mouse from the home cage by gently grasping its tail and place the mouse in the middle of the open field maze while concurrently activating the SMART software by single clicking on the Start button to begin tracking mouse movement. It is normal for the mouse to move immediately to the periphery walls of the maze and the timing of release and tracking capture of the mouse should coincide to record this movement.
- Allow free and uninterrupted movement of the subject mouse throughout the respective quadrant of the maze for a single 10 min period during which time, the tracking software will record movement (Figure 3).
- At the end of the test period, pick up the subject mouse gently, removing it from the maze and return it to its home cage.
- Prior to cleaning the maze, visually count the fecal boli pellets present in the maze and manually record the numbers for further analysis.
- Remove all fecal pellets and wipe up all spots of urination. Spray the floor and walls of the maze quadrant with 95% ethanol and wipe down with a clean paper towel. Allow the ethanol solution to completely dry prior to testing other mice.
- Repeat the procedure with the next mouse.
4. Measurement and Analysis of Behavior During Testing Procedure
NOTE: For measurement, three aspects of open field behavior are readily characterized using this protocol (see discussion). A brief instruction on how to access these measurements in the video tracking software follows.
- From the main screen of the SMART software, move the cursor to the “Zones” tab and single click “Definition” to open the Zone Editor.
- Follow the detailed instructions in the SMART software User’s Manual do define zones or grids to overlay on the tracking paths. Here, the software was used to define a 5 x 5 grid of 10 cm squares covering the floor of the maze (Figure 4). Be sure to save the zone file created prior to closing the Zone Editor.
- From the main screen of the SMART software, move the cursor to the “Analysis” tab and single click to open the Data Analysis window.
- Move the cursor to the “File” tab and open the Zone file created above.
- Move the cursor to the “Configuration” tab and open the “Track Analysis” option. This will open the “Single-subject analysis configuration” window.
- Move the cursor to the “Standard” tab and move the “Travelled distance” parameter from the Available Parameters box (left side) to the Included Parameters box (right side).
- Move the cursor to the “Zones Transitions” tab and move all appropriate parameters to the Included Parameters box as above.
- Make sure the “Full Track” box is checked at the bottom of the window.
- Move the cursor to the OK button and close the Single-subject analysis configuration window.
NOTE: Depending on the analysis you wish to perform, many other options can be chosen in this window to mine data from the analysis. Read the detailed User’s Manual of your specific program to determine what parameters are most important for analysis of your data.
- Under the File tab at the upper left of the program window, open the Single subject track window and place a check mark beside all tracks being analyzed. Move the cursor to the Check Mark button at the top of the window and click to close the Track Explorer window.
- Move the cursor to the “Go” button in the Data Analysis window and single click to initiate analysis of the track data.
- Analysis data can be output as either ASCII text files or it can be directly exported into an EXCEL spreadsheet. Use the output tools of the software program you are using to output the data for your own use.
NOTE: Total distance traveled and time spent in indicated zones will be output following the data analysis steps outlined above. Again, it is stressed that the steps to reach these measurements represented here will differ depending on the user software used. But the data itself and the interpretation of the results should be similar independent of the software program used. It is also worth noting that test administrator bias is removed from this protocol as all the data collected is quantified data measured by the software and not the administrator. Thus there is no qualifiable element to the data collected as described.
Representative Results
The average number of individuals per strain of mice tested in most cases is approximately 20 to generate sufficient statistical relevance. However, this number can be in the range of 8-30 depending on mouse availability. Depending on the measurement or comparisons required, it is also favorable to use age-matched subjects.
The first and arguably most important specific parameter to measure in the Open Field Maze is total ambulatory distance. While the unit of measure is irrelevant for comparison purposes, it is most often expressed as a metric measurement (cm). In the experimental data presented here (Figure 5), Wild type (WT) or normal C57BL/6 mice show similar ambulatory ability to a specific knock-out C57BL/6 mouse strain (KO). When total distance traversed is similar between strains or treatments, further analysis of emotional behaviors is simplified because locomotor activity is effectively removed from the equation. If there are significant differences in the locomotor ability of the mice tested, further analyses such as zone entries or time spent in certain designated zones of the maze can be skewed due to inactivity instead of strain or treatment effects. Techniques exist to account for unequal locomotor activity but these are most often specific to the research question.
As total ambulatory distance between the two mouse strains was similar, we were able to analyze thigmotaxis, or the tendency of a subject to remain close to walls, in the WT versus KO mice (Figure 6). The degree of thigmotaxis has been validated as a measure of anxiogenic behavior in mice10. Thigmotaxis increases as anxiety levels rise. Using the SMART software, individual zones were overlaid on the paths traveled by the mice (Figure 3) and time spent in inner zones versus outer zones calculated and presented as a function of total time (10 min) in the maze. In this case, KO mice displayed significantly higher anxiogenic behavior than their WT counterparts. A representative travel path can be seen in Figure 4 where the WT mouse path traverses the middle areas of the maze at a much greater frequency than does the KO mouse which remains close to the walls of the maze even though the distance traveled of both mice is similar. We can conclude from this that the KO mice exhibit higher anxiety associated behavior than do WT mice.
To further support increased anxiety levels in the KO mice, fecal boli left in the maze after the 10 min test period were counted by the observer once the test subject was removed (Figure 13). Following the popular view that highly emotional animals exhibit increased defecation, KO mice exhibit significant increases in fecal boli present when compared to WT mice. This correlates with the levels of thigmotaxis measured in the KO mice and indicates that the knock-out mice used in this study showed increased emotionality and anxiety when compared to their WT counterparts.
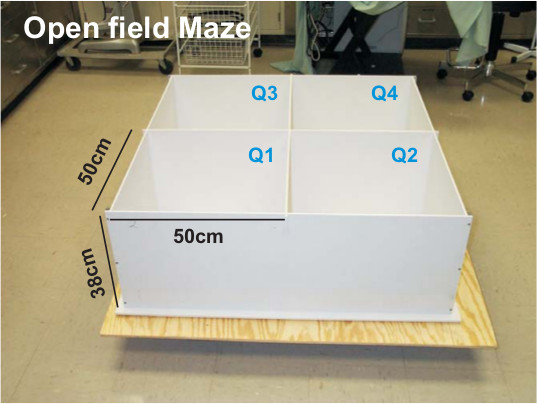
Figure 1. Example of a four quadrant OFM. The OFM pictured was obtained from San Diego Instruments and was used in all testing procedures described. Dimensions of one quadrant of the maze are in centimeters and each quadrant is identified (Q1-Q4).
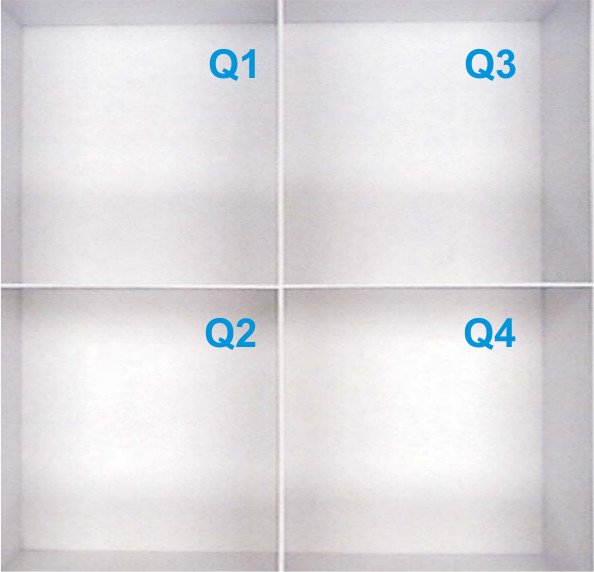
Figure 2. Example of overhead camera view of a four quadrant OFM. The image shown is representative of the camera view recognized by the SMART software prior to tracking procedures. Each quadrant is labeled (Q1) through (Q4) and would contain a single mouse for tracking.
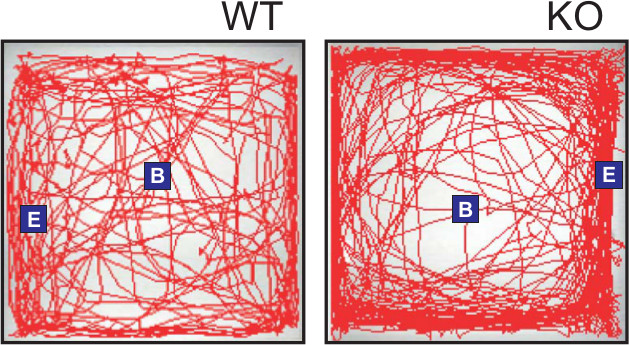
Figure 3. Comparison of track paths for WT and KO mice. Representative tracks for either WT or KO mice are shown. Each track represents the total distance traveled by the subject during the 10 min time period of the test. The beginning point (B) and the end point (E) of the tracking is indicated. The WT example track crosses into the center portion of the maze at regular intervals while the KO track remains closely in proximity to the walls of the maze indicated increased thigmotaxis or anxiety-related behavior.
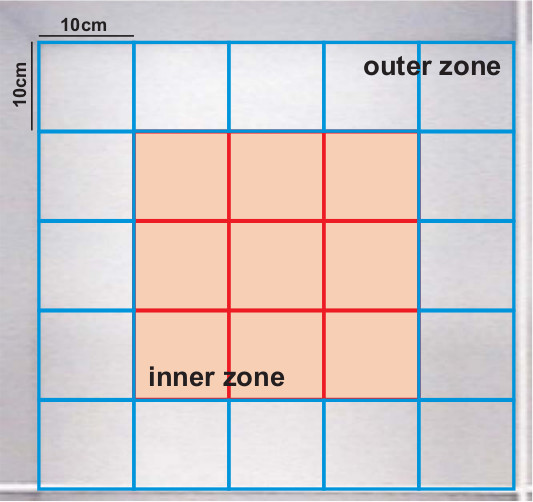
Figure 4. Zone overlay used to interpret tracking data for thigmotaxis from the OFM. Using the SMART software analysis package, a series of 10 x 10 cm zones were identified and used to evaluate subject tracks. The outer zone consisted of 16 blocks as identified while the inner zone consisted of 9 blocks and is shaded. Greater time spent in the outer zones of the maze is recorded as increased thigmotaxis and is indicative of amplified anxiety-related behavior.
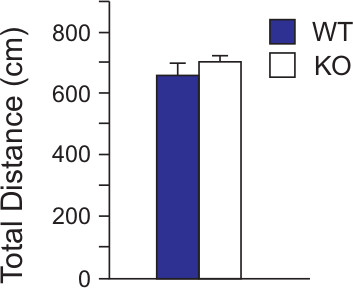
Figure 5. Total distance traveled in the OFM. WT (n = 24) and KO (n = 27) mice were subjected to the OFM and total distance in centimeters of their respective tracks were combined and statistically analyzed to visualize any differences in ambulation. WT and KO mice performed similarly in the OFM when total distance was measured. The results for the data were expressed as the mean +/- S.E.M. Statistical analyses (t-tests) were performed using Excel 2010 (Microsoft, Redmond WA) and SAS 9.2 (SAS Institute, Cary NC). [Data is modified from Ramesh Babu, et.al., 2008.]
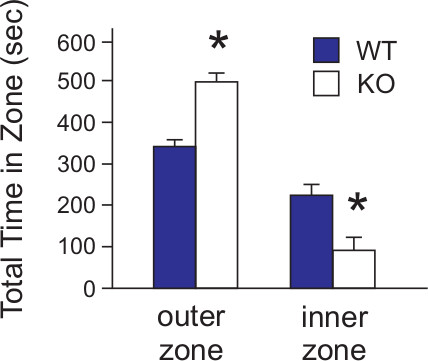
Figure 6. Time spent in inner and outer zones of the OFM. WT (n = 24) and KO (n = 27) mice were subjected to the OFM and time spent in inner and outer zones of the maze statistically analyzed for differences in mouse strains. Time spent in the outer zones of the maze identified in Figure 7 measures thigmotaxis or wall-hugging behavior and is indicative of anxiety-related behavior. KO mice exhibited higher anxiety measures than to WT based on thigmotaxis. The results for the data were expressed as the mean +/- S.E.M. Statistical analyses (t-tests) were performed using Excel 2010 (Microsoft, Redmond WA) and SAS 9.2 (SAS Institute, Cary NC). p <0.05. [Data is modified from Ramesh Babu, et.al., 2008.]
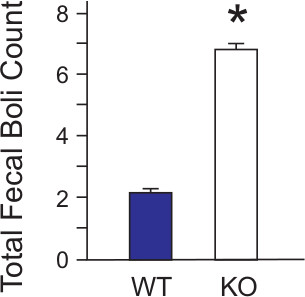
Figure 7. Fecal boli deposits in the OFM. WT (n = 24) and KO (n = 27) mice were allowed to complete a 10 min test in the OFM before being removed from the maze back to their home cages. After mice were removed, the number of defecations or fecal boli deposits was manually counted by the observer. Increased number of boli can be indicative of increased anxiety and emotionality of the subject animal. KO mice exhibited an increase in fecal boli when compared to WT. The results for the data were expressed as the mean +/- S.E.M. Statistical analyses (t-tests) were performed using Excel 2010 (Microsoft, Redmond WA) and SAS 9.2 (SAS Institute, Cary NC).p <0.05. [Data originally published in Ramesh Babu, et.al., 2008.]
Discussion
The Open Field Maze is one of the most widely used platforms in animal behavioral studies. A number of important conventional and ethological parameters2,4 can be collected and analyzed during the performance of the OFM. These data allow the researcher to measure behaviors ranging from overall locomotor activity to anxiety-related emotional behaviors8. However, use of OFM is not without its shortcomings. One confounding issue is the wide range of static variables that can be manipulated during any testing session. Examples include time, lighting conditions and novel object inclusion. Variability in experimental protocol setup and design, which are essential to support a broad-spectrum of applications, can make it difficult to compare studies. When subject variability, such as different background or transgenic mouse lines and drug treatments are included, the difficulty in test comparisons can increase even more. Despite these issues, the OFM remains one of the most widely applied techniques in rodent behavioral research. Here, we discuss results obtained from the OFM and their analysis as it relates to mouse anxiety and emotionality.
Here, three aspects of open field behavior are readily characterized using this protocol: 1) Total distance covered (in cm) during the entire timed portion of the test; 2) Thigmotaxis or a measure of the percent of the 10 min total test time that the subject remains adjacent to the outer wall of the maze which is indicative of anxiety-like behavior; 3) The number of fecal pellets (boli) left in the quadrant after the subject is removed is counted. Defecation is a negatively related measure of emotionality in rodents5 and can be used to indicate levels of anxiety in the mouse subject. A brief instruction on how to access these measurements in the SMART software follows.
Locomotor activity of the test subjects is important to discern prior to analysis of OFM data or for that matter, any animal behavioral maze. When comparing different strains of mice or different effects of drug treatments, the ambulatory ability of the mouse is paramount. If locomotor ability is compromised due to treatment effects, then measuring activities that rely on the ability of the subject to move is confounded. Therefore the first step in this experiment was to compare total movement between the two subject strains. Using the subject tracking feature of the SMART software, we measured the total ambulatory distance covered in the maze during the duration of the test by two different strains of C57BL/6 mice (Figure 4). Both Wild Type (WT) and genetic knock-out mice (KO) displayed similar ambulatory ability. There was no statistical difference in total distance (in cm) traveled by either mouse line throughout the 10 min time frame of the experiment. Had one strain shown a significant difference in ambulation compared to the other, a more specialized investigation, possibly using other behavioral paradigms, would be required to characterize the difference. However, in this case, ambulation of the subject strains was equal allowing direct use of unadjusted OFM-derived data to further investigate anxiolytic parameters. It should be noted here that some researchers have interpreted high activity or increase exploratory behavior as an index of low emotionality while others conceive of exploratory behavior being independent of emotionality11. One has to acknowledge that differences in locomotor activity can confound emotional measures12. However, as total ambulatory distance was similar between the mouse strains used here, activity levels of the mice were separated from emotionality factors.
Rearing behavior consists of subject animals standing on both hind paws in a vertical upright position. It is considered an exploratory behavior and has been used as a measure of anxiety in both the OFM and the Elevated Plus Maze13. However, there is no clear indication that rearing behavior is either anxiolytic or anxiogenic. Some studies indicate increased rearing is in concordance with increased anxiety levels in mice14 while others postulate decreased rearing behavior is indicative of increased anxiety15. Thus, while rearing was not analyzed here, depending on experimental protocol and ethological parameters examined, rearing could be used to discriminate anxiety-linked behaviors from simple ambulatory behavior.
It has been proposed that measuring anxiety in rodent models is much more complicated than using a single parameter in a single maze environment16. Therefore, using multiple tests or multiple measures in a single test can strengthen evaluation of the results. Thigmotaxis or wall-hugging behavior is observed in most rodent species and is linked to anxiety related behaviors. It is most likely tied to a rodent’s underlying propensity to avoid large open areas or areas of perceived danger17,18. Regardless of the underlying cause, thigmotaxis is an important anxiety-linked behavior that is often considered the starting point for further specific anxiety related tests19.
Thigmotaxis in the OFM is used to evaluate anxiolytic, anxiogenic and even non-pharmacological treatments. Anxiety related drugs such as diazepam and chlordiazepoxide have shown significant effects on mouse behavior in the OFM4 while dopamine agonists have shown that the D1 and D2 dopamine receptors are involved in anxiogenic-like effects due to increased dopaminergic transmissions20. There are also clear strain distinctions in response to mouse anxiety-like behavior in the OFM21. For example, BALB/c mice, an albino laboratory bred strain of the common house mouse, show greater behavioral response to acute stress than do C57BL/6 mice22. Therefore, baseline variation in responses between subjects must be taken into account when setting up the parameters associated with testing paradigms and choosing which species to use for a particular research question.
It is also the popular view that highly emotional animals exhibit increased defecation. This view has been corroborated by some, but the validity of defecation as distinct measure of anxiety has been questioned23. However, since Hall’s original treatise (1934) correlating defecation events with emotionality in rodents, a large amount of literature on the subject has affirmed this relationship2. More recent findings have indicated that defecations may indeed be a useful indicator of emotional anxiety-related behaviors in relatively short test periods as performed here as opposed to long observations (30 min) where differences in responses are less clear21.
It is important to note that behavior of mice in the OFM is dependent on their tactile sensations. Thus any damage to or shortage of whiskers to the mice may cause a decrease in measured anxiety-linked behavior as the mice lose tactile contact with the walls of the maze and enter the central portions more readily3. Maze exploration may also be dependent on food or water deprivation, lighting during the testing procedure or even on the color of the maze floor24. It is important to verify these variables, as well as any treatment induced factors, before interpreting OFM results for anxiety-related behaviors. Detailed reporting of all testing conditions in manuscripts is also critical to facilitate appropriate cross-study comparisons.
Also, while here we discuss the use of the OFM as it relates to motor locomotion and emotionality of the mice investigated, the OFM can also be used to test other behaviors such as novel object recognition and memory25. Depending on the type of memory being analyzed, time in the maze with a novel object can vary from 5 min to 24 hr. The ease and flexibility of the maze in the novel object recognition test allows for testing of short- or long-term memory, and can be used to selectively analyze the effects of acute drug treatment on a specific stage of memory formation. In conclusion, the OFM is an apical test of performance26. The anxiety-related behaviors measured are the cumulation of several behavioral underlying processes. Thus, once a response is detectably measured, it is often necessary to investigate that response further to identify a specific defect.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by The National Institute of Health (NIH-2RO1NS033661) and by the Alabama Agricultural Experiment Station (HATCH ALA021-1-09017).
Materials
| Multi Unit Open Field Test | San Diego Instruments, Inc. | White 7001-0354 | Any single or multi unit open field maze can be used |
| SMART DT Tracking Software | PanLab/Harvard Apparatus | 76-0695 | Any tracking software can be utilized with this protocol |
| Sony 990x Video Camera Recorder | Sony | CCD-TRV328 | Any suitable video camera can be attached to computer for recording tracking profiles. |
References
- Hall, C. S. Emotional behavior in the rat: defecation and urination as measures of individual differences in emotionality. J. Comp. Psychol. 18, 385-403 (1934).
- Walsh, R. N., Cummins, R. A. The open field test: a critical review. Psychol. Bull. 83, 482-504 (1976).
- Prut, L., Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. of Pharm. 463, 3-33 (2003).
- Choleris, E., Thomas, A. W., Kavaliers, M., Prato, F. S. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazpoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 25, 235-260 (2001).
- Ramos, A. Animal Models of anxiety: do I need multiple tests. TIPS. 29, 493-498 (2008).
- Archer, J. Tests for emotionality in rats and mice: a review. Anim. Behav. 21, 205-235 (1973).
- Gray, J. A. Emotionality in male and female rodents: a reply to Archer. Brit. J. Psych. 70, 425-440 (1979).
- Carola, V., D’Olimpio, F., Brunamonti, E., Mangia, F., Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behavior in inbred mice. Behav. Brain Res. 134, 49-57 (2002).
- Ramesh Babu, J., Seibenhener, M. L., Peng, J., Strom, A. L., Kemppainen, R., Cox, N., Zhu, H., Wooten, M. C., Diaz-Meco, M. T., Moscat, J., Wooten, M. W. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J. Neurochem. 106, 107-120 (2008).
- Simon, P., Dupuis, R., Costentin, J. Thigmotaxis as an index of anxiety in mice: influence of dopaminergic transmissions. Behav. Brain Res. 61, 59-64 (1994).
- Denenberg, V. H. Open-field behavior in the rat: what does it mean. Ann. N.Y. Acad. Sci. 159, 852-859 (1969).
- Stanford, S. C. The open field test: reinventing the wheel. J. Psychopharm. 21, 134-135 (2007).
- Ennaceur, A. Tests of unconditional anxiety – pitfalls and disappointments. J. Phys. Behav. 135, 55-71 (2014).
- Borta, A., Schwarting, R. K. Inhibitory avoidance, pain reactivity, and plus-maze behavior in Wistar rats with high versus low rearing activity. J. Phys. Behav. 84, 387-396 (2005).
- Costall, B., Jones, B. J., Kelly, M. E., Naylor, R. J., Tomkins, D. M. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol. Biochem. Behav. 32, 777-785 (1989).
- Bouwknecht, J. A., Paylor, R. Pitfalls in the interpretation of genetic and pharmacological effects on anxiety-like behavior in rodents. Behav. Pharm. 19, 385-402 (2008).
- Webster, D. G., Baumgardner, D. J., Dewsbury, D. A. Open field behavior in eight taxa of muriod rodents. Bull. Psychonom. Soc. 13, 90-92 (1979).
- Wilson, R. C., Vacek, T., Lanier, D. L., Dewsbury, D. A. Open field behavior in muroid rodents. Behav. Biol. 17, 495-506 (1976).
- Crawley, J. N. Behavioral phenotyping of transgenic and knockout mice: experimental designs and evaluation of general health, sensory functions, motor abilities and specific behavioral tests. Brain Res. 835, 18-26 (1999).
- Simon, P., Dupuis, R., Costentin, J. Thigmotaxis as an index of anxiety in mice. influence of dopaminergic transmissions. Behav. Brain Res. 61, 59-64 (1994).
- Miller, B. H., Schultz, L. E., Gulati, A., Su, A. I., Pletcher, M. T. Phenotypic characterization of a genetically diverse panel of mice for behavioral despair and anxiety. PLoS One. 5, e14458 (2010).
- Tannenbaum, B., Anisman, H. Impact of chronic intermittent challenges in stressor-susceptible and resilient strains of mice. Biol. Psych. 53, 292-303 (2003).
- Lister, R. G. Ethologically-based animal models of anxiety disorders. Pharmacol. Ther. 46, 321-340 (1990).
- Kulesskaya, N., Voikar, V. Assessment of mouse anxiety-like behavior in the light-dark box and open-field arena: role of equipment and procedure. Phys. Behav. 133, 30-38 (2014).
- Han, H., Du, W., Zhou, B., Zhang, W., Xu, G., Niu, R., Sun, Z. Effects of chronic fluoride exposure on object recognition memory and mRNA expression of SNARE complex in hippocampus of male mice. Biol. Trace Elem. Res. 158, 58-64 (2014).
- Barrow, P., Leconte, I. The influence of body weight on open field and swimming maze performance during the post-weaning period in the rat. Lab. Animals. 30, 22-27 (1996).

