In Situ Ca2+ Imaging of the Enteric Nervous System
Summary
The enteric nervous system (ENS) is a network of neurons and glia located in the gut wall that controls intestinal reflexes. This protocol describes methods for recording the activity of enteric neurons and glia in live preparations of ENS using Ca2+ imaging.
Abstract
Reflex behaviors of the intestine are controlled by the enteric nervous system (ENS). The ENS is an integrative network of neurons and glia in two ganglionated plexuses housed in the gut wall. Enteric neurons and enteric glia are the only cell types within the enteric ganglia. The activity of enteric neurons and glia is responsible for coordinating intestinal functions. This protocol describes methods for observing the activity of neurons and glia within the intact ENS by imaging intracellular calcium (Ca2+) transients with fluorescent indicator dyes. Our technical discussion focuses on methods for Ca2+ imaging in whole-mount preparations of the myenteric plexus from the rodent bowel. Bulk loading of ENS whole-mounts with a high-affinity Ca2+ indicator such as Fluo-4 permits measurements of Ca2+ responses in individual neurons or glial cells. These responses can be evoked repeatedly and reliably, which permits quantitative studies using pharmacological tools. Ca2+ responses in cells of the ENS are recorded using a fluorescence microscope equipped with a cooled charge-coupled device (CCD) camera. Fluorescence measurements obtained using Ca2+ imaging in whole-mount preparations offer a straightforward means of characterizing the mechanisms and potential functional consequences of Ca2+ responses in enteric neurons and glial cells.
Introduction
The enteric nervous system (ENS) is organized into two ganglionated plexuses embedded within the wall of the digestive tract 1. These intramuscular neural circuits, the myenteric plexus (MP) and submucosal plexus (SMP), are composed of neurons and enteric glia (Figure 1) 2. The MP and SMP regulate gastrointestinal (GI) functions such as intestinal motility and epithelial absorption and secretion, respectively 3. Enteric glia are located in close proximity to neurons within ganglia but populations of enteric glia also exist within interconnecting fiber tracts and extra-ganglionic portions of the gut wall 3,4. Enteric glia were originally believed to provide only nutritive support to neurons. However, recent studies strongly suggest that neuron-glia interactions are essential for ENS functions 5,6. For example, data show that enteric glia “listen” to neuronal activity 7 and modulate neuronal circuits 6,8, protect enteric neurons from oxidative stress 9 and are capable of generating new enteric neurons in response to injury 10,11. The protocol presented in this technical review provides a simple and robust method to examine the complex interplay between neurons and enteric glia using in situ intracellular Ca2+ imaging.
Ca2+ is a ubiquitous signaling molecule in excitable cells and plays an essential role in synaptic signaling events in the nervous system 12. Excitation of neurons or enteric glia elicits an elevation in cytoplasmic Ca2+ concentration either by influx through Ca2+-permeable channels or Ca2+ release from intracellular calcium stores. Imaging Ca2+ transients in neurons and glia with fluorescent dyes is an established and widely used technique to study the functional organization and dynamics of the ENS 13-17. Ca2+ imaging has been shown to be an important tool in studying intact GI tissue segments to elucidate the spread of excitability through ICC pacemaker networks 18 and gut smooth muscle 19,20. It enables researchers to probe a broad spectrum of physiological parameters and provides information about both their spatial distribution and temporal dynamics. Cells can be efficiently stained in a minimally invasive manner by using membrane-permeable fluorescent indicators and optimized staining protocols 21. This offers the opportunity to monitor a large number of neurons and enteric glia in functionally preserved preparations 14-16,22, as well as in vivo 23. Whole-mount tissue preparations are bulk loaded with a high-affinity Ca2+ indicator dye such as Fluo-4 that increases its fluorescence when bound to Ca2+. Changes in fluorescence are recorded by a CCD camera and analyzed digitally 6. The advent of Ca2+ provided the opportunity to monitor neuron and glia cell interactions, responsiveness to various stimuli, and the involvement of these cell types in gastrointestinal processes in real time.
In situ Ca2+ imaging has yielded great insight into the signaling mechanisms of enteric neurons and glia and possesses several distinct advantages over cell culture models 6,24. First, in situ preparations maintain the native matrix environment of neurons and glia and leave the bulk of their connections to target tissue intact. Second, the genetics and morphology of cultured enteric glia are significantly altered compared to in vivo 6,24. Third, many heterotypic interactions are lost in primary cell culture and this limits assessing cell-cell interactions. Although cultured cells are well suited for investigation of fundamental properties, their usefulness for studying complex interactions between enteric glia and neurons is limited. Investigating neuron-glia interplay using an in situ approach is more physiologically relevant as the synaptic pathways remain intact 25. As compared to cell culture approaches, an in situ approach offers improved conditions for systematically understanding the intricate interactions between neurons and enteric glia. Furthermore, the planar organization of the ganglionated plexus in whole-mount preparations is ideal for fluorescent imaging of intracellular Ca2+ transients and this technique provides a straightforward approach for assessing neuron-glia activity in the ENS.
Protocol
NOTE: The following procedures involving tissue from laboratory animals are consistent with the AVMA Guidelines for the Euthanasia of Animals 2013 and were approved in advance by the Michigan State University IACUC.
1. Tissue Preparation
- Anesthetize research animal in a chamber containing 2.5% isoflurane in oxygen or by placing 3-5 ml of liquid isoflurane onto an absorbent material on the floor of the chamber, ensuring that a physical barrier prevents animals from direct contact with the isoflurane. Test for the depth of anesthesia by pinching the footpad.
NOTE: The depth of anesthesia is deemed appropriate when there is no withdrawal reflex of the hind limb. Once appropriately anesthetized, euthanize the mouse by cervical dislocation - Place the animal in a supine position and clean abdominal skin with 70% ethanol. Use forceps to pinch abdominal skin at midline and use surgical scissors to make a 6 cm medial incision along the linea alba to expose internal digestive organs.
- Use blunt forceps to locate and expose the ileum inside the peritoneum. Cut the ileal/colon mesentery with scissors and begin unraveling the intestine.
- Once the length of the intestine is adequately unraveled, cut the intestine distal to the stomach and proximal to the cecum for an ileum preparation. For a large intestine preparation, cut the colon distal to the cecum and proximal to the rectum.
- Quickly remove the intestinal segment and place it in a beaker with DMEM/F12 media supplemented with 3 μM nicardipine hydrochloride and 1 μM scopolamine hydrochloride (hereafter referred to as “media”) on ice. The addition of these inhibitors facilitates microdissection and subsequent imaging by paralyzing the gut smooth muscle.
- Cut segment of interest (e.g., jejunum, ileum, distal or proximal colon) based on established anatomical markers. Typically utilize tissue from the distal ileum or distal colon. However, use the same basic procedure to isolate, load and image myenteric neurons and glia in all intestinal regions.
- Remove a small segment (4-6 cm) of desired intestine segment and place in a Sylgard–coated petri dish filled with chilled media.
- Secure the proximal and distal ends of the intestinal segment with insect pins and open the intestinal tube by making a straight, lengthwise cut along the mesenteric border.
- Pin tissue flat under light tension with mucosa side up and carefully dissect away mucosal layer using fine forceps (#5 and #5/45 work best) and very fine spring scissors.
NOTE: The removal of the mucosa can be quite traumatic for the ENS if not done properly. For quality preparations, take care to limit abrupt removal of the mucosa by peeling or scraping. The best practice is to lift the mucosa and cut underneath with fine scissors. - Cut the tissue into smaller preparations (approximately 0.5 cm2) and pin into imaging dishes (4 corners with circular muscle layer facing up) placed on ice with fresh media.
- Carefully dissect away circular muscle by teasing apart with fine tweezers to expose the myenteric plexus. Avoid excessive stretching.
- Place imaging dish back on ice and change solution with fresh media.
- Prepare 2 ml of enzyme mix per dish [Dispase 1 U/ml (4.48 mg/8 ml), Collagenase Type II 150 U/ml (5.45 mg/8 ml) in media].
- Remove dishes from ice and add the enzyme mix from step 1.13.
- Incubate dishes at RT for 15 min with 5% CO2 / 95% air.
- Wash tissue preparations with media 3 times and re-pin corners.
2. Loading Fluo-4 Dye
NOTE: Avoid photobleaching by working with limited light while handling fluorescent dyes and tissue loaded with indicator dyes.
- Prepare 4 μM Fluo-4 loading solution.
- Add 1.5 ml media and 1.2 μl of a 250 mM probenecid stock to a 1.5 μl aliquot of 4 mM Fluo-4 stock. 4 mM Fluo-4 stock is prepared by adding 11.4 μl of pluronic F-127 (20% in DMSO; supplemented with 0.25% cremaphor-EL) to 50 μg of Fluo-4, AM.
- Incubate preps in Fluo-4 loading solution for 45 min in a dark incubator at 37 °C.
- Remove from incubator and wash preparations with media 3 times.
- Exchange media for media containing 200 μM probenecid and incubate 15 min at 37 °C before imaging.
NOTE: Probenecid is a drug that inhibits multidrug resistance transporters in neurons. Addition of this drug inhibits the ability of neurons to extrude dyes and enhances neuronal labeling. Bulk-loading of Ca2+ indicator dyes in the absence of probenecid produces mainly glial cell loading. The addition of probenecid allows for visualization of neuronal and glial responses. - Prepare Modified Krebs buffer.
- Make a modified Krebs buffer such that the final concentrations (in mM) of the components are as followed: 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 10 HEPES, 21.2 NaHCO3, 1 pyruvic acid and 8 glucose (pH adjusted to 7.4 with NaOH). Add 3 μM nicardipine and 1 μM scopolamine to inhibit muscle contractions during Ca2+ imaging and whole-mount dissections.
3. Imaging and Analysis
NOTE: Use at least a basic imaging rig with a fluorescent light source, microscope, a quality CCD camera and appropriate acquisition software. Vary the addition of other components depending on the light source and specific application. A filter wheel and shutter must be used with a traditional xenon arc light source. However, LED light sources and illumination systems do not require those components.
- Position the recording chamber under the microscope and using a gravity flow perfusion system with multiple heated syringe reservoirs establish a continuous perfusion rate of 2–3 ml/min of 37 °C Krebs buffer. Make sure to prevent air bubble formation in both the input and suction line connected to a vacuum trap.
- Bring the desired plexus into focus under bright field illumination. Avoid overexposing tissue, which may lead to photo bleaching.
- Examine the Fluo-4 loading within ganglia and select healthy ganglia for imaging. Unhealthy/damaged ganglia will exhibit autofluorescence or punctate morphology and should not be used for imaging.
- Once ganglion is selected, divert light path to camera and obtain live image with image acquisition software. Ensure that ganglion is in focus and set image acquisition rate and exposure times.
NOTE: Image acquisition rates and times will vary depending on the events investigators wish to record. For most experiments, images are traditionally acquired at 0.5-1 Hz for glial cells and up to 2-10 Hz for neurons because glial Ca2+ responses are not as rapid as Ca2+ transients in neurons. - Begin experiment and establish baseline activity for 30 sec.
- Apply pre-warmed drugs of interest such as receptor agonists and antagonists using the gravity flow perfusion system at a rate of 2-3 ml/min. Follow application of agonists/antagonists by returning to a perfusion of normal buffer and allow a wash/recovery period of at least 10 min.
NOTE: The drug application times will vary depending on the individual compound and experimental design. In general, a 20-30 sec application of agonist is sufficient to activate G-protein coupled receptors in neurons and glial cells. However, ligand-gated ion channels (such as nicotinic acetylcholine receptors) require application times of no more than 5-10 sec. Further duration exposures will cause ligand gated ion channels to rapidly desensitize. Antagonists should be applied for approximately 3-15 min to ensure complete blockade of receptor pathways. However, this is a gross generalization and investigators should always optimize any experimental drug in their particular paradigm. - Stop recording and view time-lapse movie of the experiment. Carefully select regions of interest (ROI’s) using the appropriate image analysis software.
- Use software to normalize and compare ROI fluorescent intensity against its initial baseline fluorescent value. Changes in normalized fluorescence are directly proportional to changes in [Ca2+].
- Use a modification of a method described previously 26 using ΔF/F = ((F1− F0)/F0)ROI − ((F1− F0)/F0)background, where F1 is the fluorescence at any given point and F0 is the baseline fluorescence, to improve the assessment accuracy. This modification aids in reducing noise from fluorescence changes in tissue preparations that exhibit movement of the muscle layer underlying the myenteric plexus.
Representative Results
Proper use of this technique allows investigators to accurately measure intracellular Ca2+ [Ca2+]i transients in enteric neurons and glia in whole-mount tissue preparations. A representative example of an agonist-evoked Ca2+ responses in glia within a myenteric ganglion from the mouse colon is shown in Video 1. The following results are meant to illustrate some representative results we have obtained using this method. First, Figure 2 illustrates the results of an experiment measuring enteric glial [Ca2+]i changes in response to stimulation by ATP within guinea pig colonic longitudinal myenteric muscle plexus (LMMP) preparations. Specifically, this figure shows the method for proper analysis of the experimental protocol listed above including the outline of the analyzed myenteric ganglion and asterisks denoting the location of enteric neurons. These results also illustrate the optimal dose of one hundred micromolar ATP on the mobilization of [Ca2+]i in guinea pig myenteric glia. This response may be used to calibrate enteric glial stimulation and normalize responses to test stimuli. Next, Figure 3 elucidates how to properly select regions of interest (ROIs) surrounding glial cells, shown with surrounding yellow circles. These results also show the desired fluorescence changes under basal conditions and in response to pharmacological stimuli. Finally, Figure 4 demonstrates the spatial considerations for choosing enteric glia and neurons for [Ca2+]i responses in whole-mount preparations.
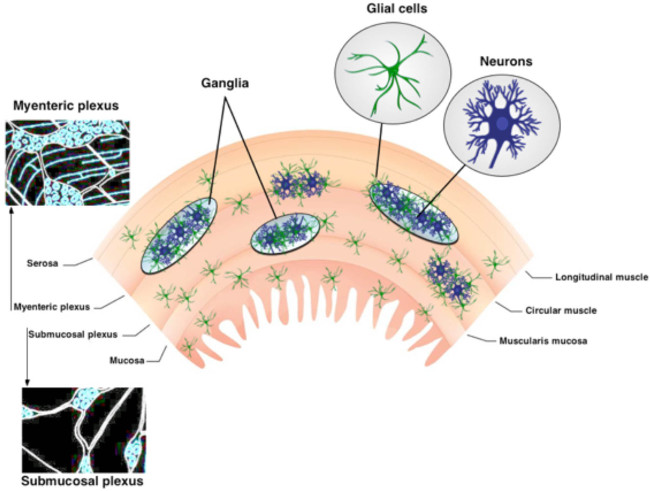
Figure 1. Organization of the ENS. The ENS contains two major ganglionated plexuses. The myenteric plexus is located between the longitudinal and circular muscle layers. The submucosal plexus is situated between the mucosa and the circular muscle layer. The ENS is solely comprised of neurons and enteric glia. Nerve fiber tracts connect the ganglia. Please click here to view a larger version of this figure.
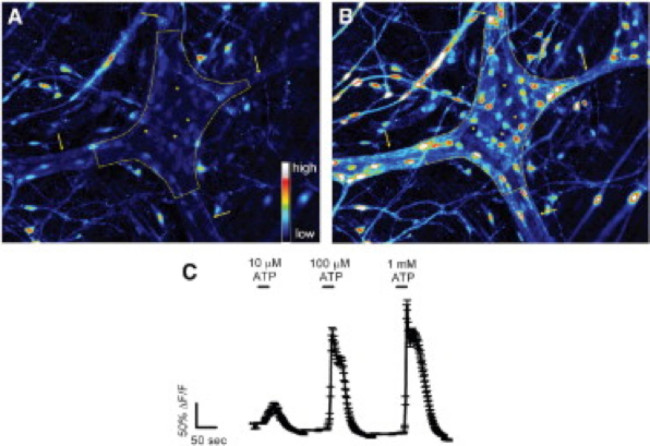
Figure 2. Enteric glia in the guinea pig colonic myenteric plexus respond to ATP in situ. (A) Fluo-4 fluorescence in a myenteric ganglion (outlined by dashed line) under basal conditions. Arrows point to thick interganglionic fiber tracts. (B) Upon stimulation with 100 μmol/L ATP, glial cells, but not neurons, rapidly increase Fluo-4 fluorescence indicating an increase in [Ca2+]i. Note that responding cells are small and surround the much larger neurons (dark spaces marked by asterisks). (C) Enteric glia respond to ATP in a dose-dependent manner with 1 mmol/L eliciting maximal responses 24. Please click here to view a larger version of this figure.
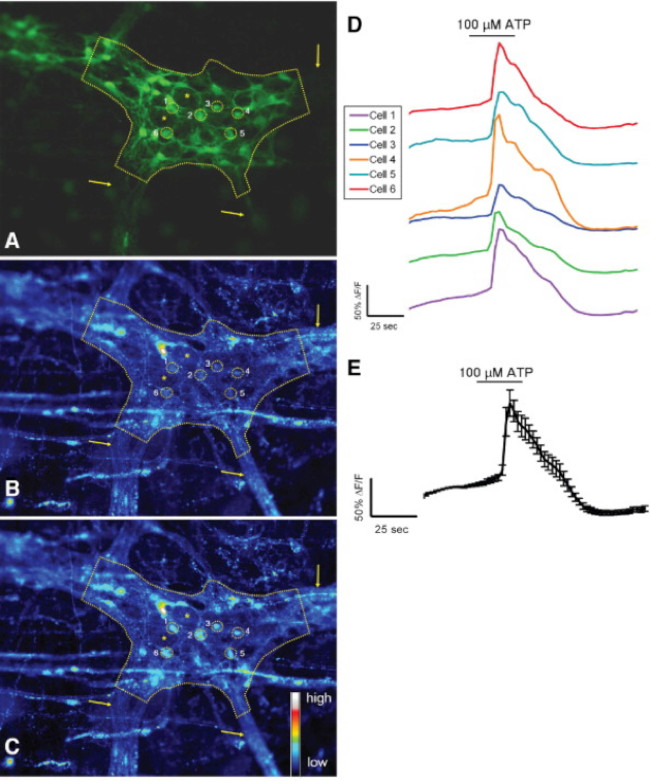
Figure 3. Murine S-100-GFP+ cells in the colonic myenteric plexus respond to ATP in situ. (A) S-100-GFP+ glial cells (green) in a mouse colonic myenteric ganglion (outlined by dashed line). Six regions of interest (ROIs) surrounding glial cells within the ganglia are shown as yellow circles. Arrows indicate thick fiber tracts leading into the ganglion. Asterisks denote location of 2 enteric neurons. (B) Same ganglion showing Rhod-2 fluorescence under basal conditions. (C) Following stimulation with 100 μmol/L ATP, glial cells respond with increased [Ca2+]i as shown by increased Rhod-2 fluorescence. (D) Traces corresponding to each ROI shown in A–C. (E) Averaged response (mean ± SEM) of the 6 ROIs shown in D 24. Please click here to view a larger version of this figure.
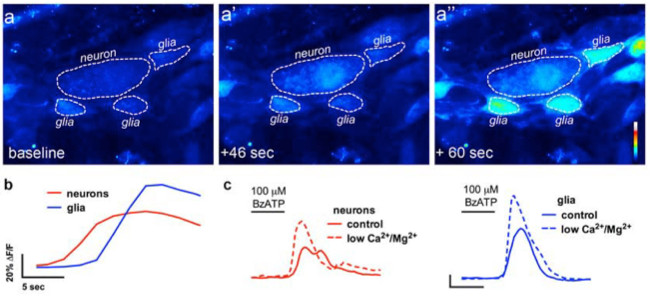
Figure 4. In situ imaging of enteric neuron-to-glia communication. (A) Representative images (pseudocolored) from a Ca2+ imaging experiment where a whole-mount preparation of the myenteric plexus was challenged with the neuronal P2X7 receptor agonist BzATP (100 μM, 30 sec). Note that the neuronal agonist causes an increase in Fluo4 fluorescence in the neuron (A’) prior to the surrounding enteric glial cells (A”). (B) Analysis of the change in fluorescence over time in glia (blue) and neurons (red) following application of the neuronal agonist, BzATP. (C) Neuronal and glial responses to BzATP in normal buffer (solid lines) and in buffer containing low Ca2+ and Mg2+ (dashed lines) to potentiate neuronal P2X7 receptors 13. Please click here to view a larger version of this figure.
Video 1. Agonist-evoked Ca2+ response in enteric glia in situ. This video shows a myenteric ganglion from the mouse distal colon loaded with the Ca2+ indicator dye, Fluo-4. The glial cell agonist, ADP, is added to the bath when indicated. ADP elicits an increase in intracellular Ca2+ in enteric glia as observed by the transient elevation in Fluo-4 fluorescence. Please click here to view this video.
Discussion
The methodologies described in this manuscript provide a consistent approach to effectively study neurons and enteric glia in the ENS. Although imaging neurons and enteric glia in culture has yielded a wealth of insight into the function of individual cells, studying these cells in their native, multi-cellular environment is crucial for our understanding their physiology and pathophysiology. Fluorescence microscopy is a crucial technique for assessing multidirectional interactions of cells in the ENS. Loading cells of the ENS with selective fluorescent markers and image acquisition with high-resolution microscopy permits quantitative observations of cellular activity in the ENS. Imaging live tissue samples of the ENS is performed relatively quickly and generates large amounts of in-depth functional and spatial data. Mouse myenteric and submucosal plexus preparations used in these experiments allow for molecular and genetic manipulation approaches. Ca2+ imaging in whole-mount preparations provides a useful tool for the assessment of neuron-glia interactions.
In advanced experimental paradigms, several probes can be combined to obtain information about different events within the cells. Fluorescence microscopy can record images with enhanced contrast of specific molecules, if an appropriate fluorescent label is used. Fluo-4 was chosen because it possesses a large dynamic range. Sufficient incubation time is vital when using the AM dyes in ENS. Dye concentration and loading method may need to be adjusted to achieve best results. Ideal preparations should be loaded with sufficient dye to visualize changes in fluorescence but not so much so that the dye chelates the target ions and interferes with intracellular signaling. Exposure to fluorescent light should be limited to prevent phototoxicity in cells and photobleaching of dyes.
Investigators must be careful with several steps of this experiment, especially solution and tissue preparation. Particular care has to be taken during processing and dissection of ENS tissue in order to maintain cellular functions. The GI tract contains several layers and tissue varieties, which pose challenges for dissection and imaging quality in these whole-mount preparations 27. Furthermore, the interconnecting fiber tracts of the MP are wider and ganglia are larger than those of the SMP 2. The neuronal density of the myenteric plexus is higher compared to that of the submucosal plexus 28. Slow and imprecise dissections will have detrimental effects on the quality of the plexus preparations and thus the overall success of the experiments. Therefore, clean/undamaged tools, practice and manual dexterity are critical to this procedure.
In whole-mount tissue preparations, careful consideration should be taken when drawing the regions of interest (ROI) to correctly assess the kinetics and degree of observed change in fluorescence intensity of the desired cell type. As the ganglia are located on a contractile muscle layer, motion artifacts caused by gut motility are a primary concern during in situ imaging. Thus, suppressing these motion disturbances through re-pinning tissue preparations after incubation with enzymes and the addition of pharmacological inhibition (nicardipine/scopolamine) to buffers permits clear and reliable image acquisition. Aside from pharmacology and mechanical approaches to prevent tissue movement, recent studies illustrate the application of advanced software methodologies and cell type response characteristics to correct for residual tissue movement in the recordings and improve the accuracy of analysis 29. Barring these technical hurdles, this method provides physiologically relevant conditions to assess morphologic and quantitative characteristics of neurons and enteric glia in the ENS.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the Pharmaceutical Research and Manufacturers Association of America (PhRMA) Foundation (to B. Gulbransen), National Institutes of Health (Building Interdisciplinary Research Careers in Women’s Health) grant K12 HD065879 (B. Gulbransen) and start-up funds from Michigan State University (B. Gulbransen).
Materials
| Name | Company | Catalog Number |
| BubbleStop Syringe Heater | AutoMate Scientific | 10-4-35-G |
| CaCl2 | Sigma | C3306 |
| Collagenase, Type II, powder | Gibco | 17101-015 |
| Dispase | Sigma-Aldrich | 42613-33-2 |
| Dissection tools | Roboz | |
| DMSO | Sigma-Aldrich | D5879 |
| Fixed-stage microscope | Olympus | BX51WI |
| Fluo-4 AM dye | Invitrogen | F-14201 |
| Glucose | Sigma | G8270 |
| Insect pins | Fine Science Tools | Minutien Pins |
| iQ Live Cell Imaging Software | Andor | Andor iQ3 |
| KCl | Sigma | P3911 |
| MgCl2 | Sigma | M9272 |
| NaCl | Sigma | S9888 |
| NaH2PO4 | Sigma | S8282 |
| NaHCO3 | Sigma | S6014 |
| Neo sCMOS camera | Andor | Neo 5.5 sCMOS |
| Nicardipine | Sigma | N7510 |
| Perfusion chamber | Custom | |
| Peristaltic pump | Harvard Apparatus | Model 720 |
| Pluronic F-127 | Invitrogen | P3000MP |
| Probenecid | Molecular Probes | P36400 |
| Scopolamine | Sigma | S1013 |
| Sutter Lambda DG-4 | Sutter | DG-4 |
| Sylgard | Dow Corning | 184 |
| Temperature Controller | Warner Instruments | TC-344C |
References
- Furness, J. B. Types of neurons in the enteric nervous system. Journal of the Autonomic Nervous System. 81 (1-3), 87-96 (2000).
- Furness, J. B. The organization of the autonomic nervous system: peripheral connections. Neuroscience. 130 (1-2), 1-5 (2006).
- Pham, T. D., Gershon, M. D., Rothman, T. P. Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. Journal of Comparative Neurology. 314 (4), 789-798 (1991).
- Nasser, Y., et al. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am J Physiol Gastrointest Liver Physiol. 291, G912-927 (2006).
- . Glial cells in the gut. Neurogastroenterology & Motility. 17 (6), 777-790 (2005).
- Broadhead, M. J., Bayguinov, P. O., Okamoto, T., Heredia, D. J., Smith, T. K. Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J. Physiol. 590, 335-350 (2012).
- Gulbransen, B. D., Bains, J. S., Sharkey, K. A. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J. Neurosci. 30, 6801-6809 (2010).
- McClain, J. L., et al. Ca2+ Responses in Enteric Glia Are Mediated by Connexin-43 Hemichannels and Modulate Colonic Transit in Mice. Gastroenterology. 146 (2), 497-507 (2014).
- Chandrasekharan, B., et al. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterology & Motility. 23 (2), e131-e126 (2011).
- Abdo, H., et al. Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J. 24, 1082-1092 (2010).
- Aube, A. C., et al. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut. 55, 630-637 (2006).
- Berridge, M. J., Lipp, P., Bootman, M. D. The versatility and universality of calcium signalling. Nature Reviews Molecular cell biology. 1 (1), 11-21 (2000).
- Gulbransen, B. D., et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 18, 600-604 (2012).
- Bayguinov, P. O., Hennig, G. W., Smith, T. K. Calcium activity in different classes of myenteric neurons underlying the migrating motor complex in the murine colon. J Physiol. 588, 399-421 (2010).
- Okamoto, T., Bayguinov, P. O., Broadhead, M. J., Smith, T. K. Ca(2+) transients in submucous neurons during the colonic migrating motor complex in the isolated murine large intestine. Neurogastroenterol Motil. 24 (8), 769-778 (2012).
- Kunze, W. A., Clerc, N., Furness, J. B., Gola, M. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differentially to deformation. J Physiol. 526, 375-385 (2000).
- Schemann, M., Michel, K., Peters, S., Bischoff, S. C., Neunlist, M. Imaging and the gastrointestinal tract: mapping the human enteric nervous system. Am J Physiol. 282, G919-G925 (2002).
- Hennig, G. W., et al. Visualization of spread of pacemaker activity in through ICC in guinea-pig antrum. Neurogastro Motil. 14, 575 (2001).
- Stevens, R. J., Publicover, N. G., Smith, T. K. Induction and organization of Ca2+ waves by enteric neural reflexes. Nature. 399, 62-66 (1999).
- Stevens, R. J., Publicover, N. G., Smith, T. K. Propagation and neural regulation of calcium waves in longitudinal and circular muscle layers of guinea-pig small intestine. Gastroenterology. 118, 982-984 (2000).
- Jessen, K. R., et al. Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. Journal of Neuroscience. 3 (11), 2206-2218 (1983).
- Gulbransen, B. D., Sharkey, K. A. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 9, 625-632 (2012).
- Gomes, P., et al. ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterology & Motility. 21 (8), e870-e862 (2009).
- Gulbransen, B. D., Sharkey, K. A. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 136, 1349-1358 (2009).
- Ren, J., Bertrand, P. P. Purinergic receptors and synaptic transmission in enteric neurons. Purinergic Signal. 4, 255-266 (2008).
- Takahashi, A., Camacho, P., Lechleiter, J. D., Herman, B. Measurement of intracellular calcium. Physiol Rev. 79, 1089-1125 (1999).
- Dongcheng, Z., et al. Neural crest regionalisation for enteric nervous system formation: implications for Hirschsprung’s disease and stem cell therapy. Developmental Biology. 339 (2), 280-294 (2010).
- Gershon, M. D. Behind an enteric neuron there may lie a glial cell. J Clin Invest. 121, 3386-3389 (2011).
- Boesmans, W., et al. Imaging neuron-glia interactions in the enteric nervous system. Frontiers in Cellular Neuroscience. 7, (2013).

