Iridium Oxide-reduced Graphene Oxide Nanohybrid Thin Film Modified Screen-printed Electrodes as Disposable Electrochemical Paper Microfluidic pH Sensors
Summary
The study demonstrates the growth of iridium oxide-reduced graphene oxide (IrO2-RGO) nanohybrid thin films on irregular and rough screen-printed carbon substrate through a green electrochemical synthesis, and their implementation as a pH sensor with a patterned paper-fluidic platform.
Abstract
A facile, controllable, inexpensive and green electrochemical synthesis of IrO2-graphene nanohybrid thin films is developed to fabricate an easy-to-use integrated paper microfluidic electrochemical pH sensor for resource-limited settings. Taking advantages from both pH meters and strips, the pH sensing platform is composed of hydrophobic barrier-patterned paper micropad (µPAD) using polydimethylsiloxane (PDMS), screen-printed electrode (SPE) modified with IrO2-graphene films and molded acrylonitrile butadiene styrene (ABS) plastic holder. Repetitive cathodic potential cycling was employed for graphene oxide (GO) reduction which can completely remove electrochemically unstable oxygenated groups and generate a 2D defect-free homogeneous graphene thin film with excellent stability and electronic properties. A uniform and smooth IrO2 film in nanoscale grain size is anodically electrodeposited onto the graphene film, without any observable cracks. The resulting IrO2-RGO electrode showed slightly super-Nernstian responses from pH 2-12 in Britton-Robinson (B-R) buffers with good linearity, small hysteresis, low response time and reproducibility in different buffers, as well as low sensitivities to different interfering ionic species and dissolved oxygen. A simple portable digital pH meter is fabricated, whose signal is measured with a multimeter, using high input-impedance operational amplifier and consumer batteries. The pH values measured with the portable electrochemical paper-microfluidic pH sensors were consistent with those measured using a commercial laboratory pH meter with a glass electrode.
Introduction
The determination of pH is ubiquitous in food, physiological, medicinal and environmental studies. Two most common tools for pH detection are pH strips and pH meters. Paper strips are impregnated with color-changing pH indicator molecules but the reading is sometimes limited in pH ranges, subjective and semi-quantitative with some deviations. On the other hand, a pH meter conventionally equipped with a glass electrode can measure pH accurately to the 0.01 level, and display by a digital-user interface. Lab-based pH meters not only need special care in maintenance and calibration, but also do not work well towards small sample volumes and often require a clean container such as a beaker to perform measurements. In spite of its sensitivity, selectivity and stability, glass electrodes suffer from acid/alkaline errors, high impedance, temperature instability and mechanical fragility1. Therefore it is advantageous to have a pH measurement system that embodies the accuracy of pH meter and the simplicity and cost aspects of pH strips.
There is always an unmet need for such tools under limited resources conditions in many developing regions where expensive lab-based equipment or commercial laboratories are unaffordable. Also, the increasing role of new easy-to-use on-site sensing platforms is pushed by such a demand for point-of-care detection. Electrochemical detection is simple, easy to miniaturize and satisfactorily sensitive, as demonstrated by the commercialized low-cost SPEs and various glucose monitoring systems on the market. As a light, flexible and disposable porous material, paper can also have various controllable characteristics, such as different pore sizes, functional groups, and wicking rates.
As paper substrate barely affects analyte diffusion and electrochemical detection2-4, combination of paper-fluidic devices and electroanalytical techniques has recently received extensive interests. An apparent advantage of such combinations is the tiny amount of sample volume used in the measurement which can potentially prevent interferences from vibration and convection during measurements. For instance, patterned microfluidic pads were applied to wick and deliver liquid samples to sensing area of SPEs for detection of heavy metal ions and glucose2,5. Similar devices using paper microfluidic electrochemiluminescence were established to accomplish NADH detection4. More recently, simple electrochemical paper microfluidic devices can be built on a glass slide with pencil electrodes6 or using enzyme paper and SPEs3.
A nanohybrid thin film material composed of IrO2 and RGO was prepared using a facile and efficient electrochemical approach. We found that on the irregular and rough SPE graphitic carbon surface, anodically electrodeposited IrO2 thin film cannot be smooth and stable without the aid of RGO. The resulting IrO2-RGO SPE was integrated into a paper microfluidic device which has patterned hydrophobic barriers for pH sensing. The assembled device showed excellent analytical performances in pH sensing with a slightly super-Nernstian behavior. The results are comparable to a conventional lab-based pH meter with glass electrodes. Lastly, cost-effective miniaturized pH meters were built on a breadboard to measure open circuit potential output signal with a digital multimeter. The measurements of the portable pH meter correlates well with those of a commercial laboratory pH meter.
Protocol
1. µPAD and Apparatus Preparation
- Engrave a 500 µm groove on the bottom plastic holder to house SPE with an ABS or compatible plastic sheet by three-dimensional (3D) milling machine and milling bit which has 1.6 mm of diameter. Hold SPE and µPAD firmly in place during testing with the holder (Figure 1A).
- Make a stamp and a vacuum cover using synthetic resin tablet or compatible plastic sheet with convex and concave patterns, respectively, by the 3D milling machine, in order to pattern hydrophobic PDMS barriers on paper pads.
- Prepare a mixture of PDMS pre-polymer and cross linker at the ratio of 10:1 or as suggested by manufacturer, mix with spatula and apply appropriate amount onto the convex surface of the PDMS stamp.
- Place the stamp on top of a filter paper pad pre-cut to desired size and then the vacuum cover on the opposite side of the stamp across the paper. Apply vacuum for up to 30 sec by a hand-operated vacuum pump. Remove the paper pad from the stamp and vacuum cover, and bake in a convection oven for 10 min at 80 °C to harden the patterned PDMS (Figure 1B). The resulting paper pad has an approximately 0.2 cm2 sensing region and 1 cm x 0.4 cm hydrophilic sample wicking region.
Note: Take special cautions on the amount of applied PDMS and vacuum time to avoid any possible PDMS contamination in the inner hydrophilic region of the filter paper where the liquid samples are transferred.
2. Modification of SPEs with IrO2-RGO Nanohybrid Thin Films
- Drop cast 3 μl of as-prepared 1 mg∙ml-1 GO solution on the graphitic carbon working electrode of SPE with a micropipette and let it dry at room temperature in a Petri dish. Purge a pH 5.0 PBS buffer with N2 for 20 min, dip the SPE in the 10 ml deaerated PBS buffer while keeping N2 flowing, and conduct 100 cycles of repetitive cathodic potential cycling from 0.0 to -1.5 V to electrochemically reduce GO into RGO. Rinse the SPE with DI water in a squirt bottle and dry at room temperature.
Note: Well-exfoliated GO sheets, stabilized by electrostatic repulsion, are from graphite powder using modified Hummer's method as reported elsewhere7. The homogeneity of as-synthesized RGO film is important, because it serves as the carbon support for further growth of IrO2 thin films. - Make 100 ml IrO2 deposition solution composed of 0.15 g iridium tetrachloride (IrCl4), 0.6 ml 50% (w/w) hydrogen peroxide (H2O2) and 0.5 g oxalic acid dehydrate by adding them in DI water. Gradually add small amount of anhydrous potassium carbonate while stirring until the pH reached 10.5, verified by a lab-based pH meter. Then, solution turned yellowish. Aging the solution for 48 hr at room temperature, then its color is eventually turning pale blue.
- Put the RGO-SPE in the above deposition solution and apply a constant potential of +0.6 V for 5 min. The thickness of IrO2 thin films can be precisely controlled by the deposition potential and time.
- Confirm the structure of the sensing area by SEM. Acquire SEM images following instructions at the Materials Science Center in University of Wisconsin-Madison, as we did before7.
3. Construction of Inexpensive and Portable Digital pH Meters
- Build an inexpensive and miniaturized pH meter with digital display by plugging in either a series of two single LF356N operational amplifiers (OpAmps) or one INA111 high speed field-effect transistor (FET)-input instrumentation amplifier (high input impedance > 1012 Ω) on breadboard to achieve sufficiently high internal impedance for stable measurements.
Note: All the parts are easily accessible from electronic stores and can be easily assembled. - Use the IrO2-RGO-SPE as the pH probe and OpAmps as the unity gain buffer. Connect two grounded 9 V alkaline consumer batteries in series to power the pH meter and plug in the wires into the breadboard based on the pin layout of OpAmps.
- Connect the cathode and anode to pins 7 and 4. Also connect the positive and negative probes of a digital multimeter to pins 6 and 5 of OpAmps respectively to measure the output voltage and display readings. Reference and working electrodes of SPE are connected to pins 2 and 3 correspondingly. Detailed connections are shown in Figure 1D.
4. pH Measurements
- Prepare 100 ml B-R buffers with 0.04 M equimolar phosphoric acid, acetic acid and boric acid and mix with different volumes (5, 25, 42, 60, 78 and 98) of 0.2 M sodium hydroxide (NaOH) to achieve different pHs from 2-12 for calibration.
- Locate patterned μPAD on top of the sensing area. Mount 60 µl liquid samples directly by a micropipette into the hydrophilic area of the μPAD for wicking. The μPAD can be held in place with or without ABS cover, when it is wetted.
- Measure the voltage signal between the IrO2-RGO working electrode and the Ag/AgCl reference electrode over time with either a lab-based CHI 660D electrochemical analyzer or the portable digital pH meter, when the open circuit potentials (OCP) become steady (potential variations < 5%).
- Keep the sensing region wet by immersing the paper pad in liquid samples to be tested, if needed, to achieve better electrical contact as well as stable and reproducible readings in long-term operation. Recorded steady-state OCP values are averaged at each pH value to determine a calibration curve.
Representative Results
The setup of the electrochemical IrO2-RGO-SPE pH sensor incorporating paper microfluidics is shown in Figure 1A. The patterned paper pad with PDMS hydrophobic barriers was placed on top of the sensing area of IrO2-RGO-SPE which located on the ABS plastic holder. The sensing zone of paper pad was carefully aligned with electrode surface. An aqueous methylene blue dye solution was used to test the patterned paper pad and as observed, samples wick into the hydrophilic regions (Figure 1B) with the fluidic route regulated by the hydrophobic barriers. SEM images shows a formation of 2D defect-free homogeneous graphene thin film by the electrochemical reduction technique, and also a synthesis of uniform and smooth IrO2 film without no observable cracks by electrodeposition (Figure 2A and C). The resulting IrO2-RGO electrode showed slightly super-Nernstian responses from pH 2-12 in Britton-Robinson (B-R) buffers with good linearity in both of bulk solution and paper (Figure 3A), small hysteresis widths (Figure 4B) and low sensitivities to dissolved oxygen (Figure 3B). The pH values measured with the portable electrochemical paper-microfluidic pH sensors were consistent with commercial laboratory pH meter using a glass electrode (Figure 5A).
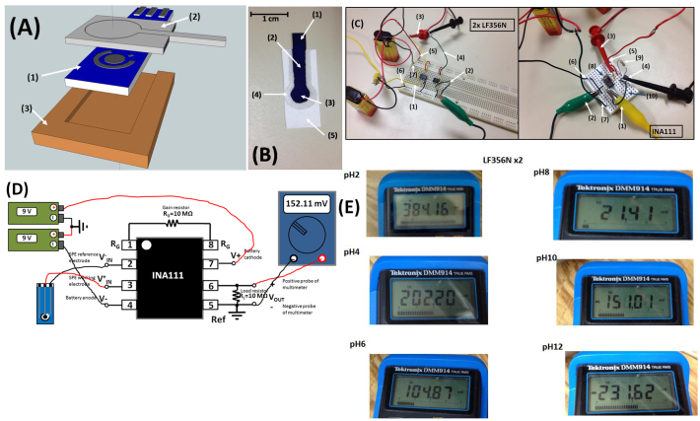
Figure 1: (A) Schematic diagram of the setup for electrochemical paper microfluidic pH sensing: (1) IrO2-RGO-SPE, (2) microfluidic paper pad for sampling and detection, (3) ABS plastic holder housing the SPE. (B) Photograph of the microfluidic paper pad wicked by a dye solution: (1) hydrophilic sampling region for wicking (2) paper microfluidic channel for sample delivery (3) sensing region in SPEs (4) hydrophobic barriers patterned by PDMS (5) region to hold device. (C) Two portable pH measuring devices with different circuits built on breadboard (detailed circuits are shown in supplementary information): (1) working electrode (2) reference electrode (3) positive probe (4) negative probe of a multimeter (5) battery cathode (6) anode (7) battery grounded (8) 10 MΩ gain resistor (9) 10 MΩ load resistor (10) grounded. (D) Connection diagrams of the portable electrochemical paper fluidic pH sensor built with INA111 and IrO2-RGO-SPE. (E) Typical digital readings of open circuit potentials using the portable electrochemical paper microfluidic pH device with LF365N integrated circuits at different pH values. Please click here to view a larger version of this figure.
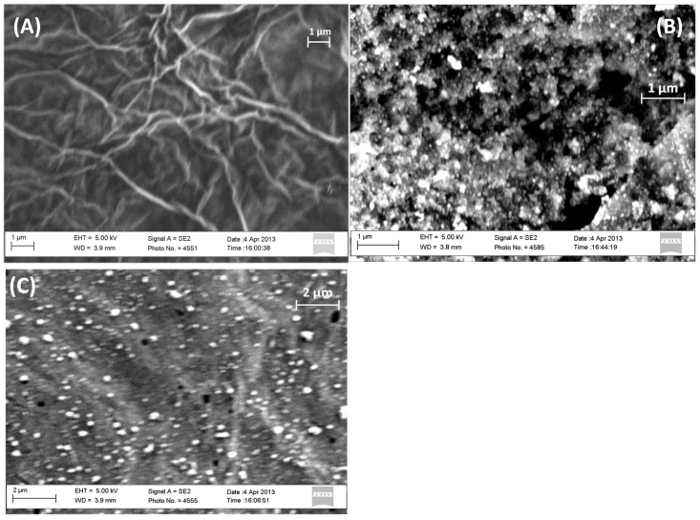
Figure 2: SEM images of (A) RGO (B) IrO2 and (C) RGO-IrO2-modified SPEs. Please click here to view a larger version of this figure.
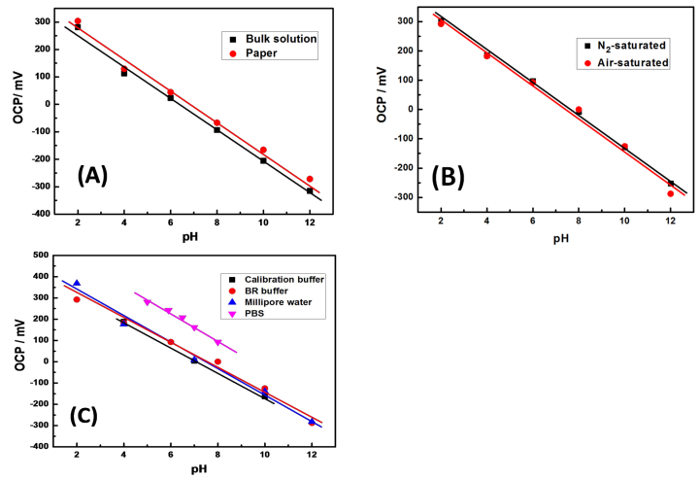
Figure 3: pH responses of RGO-IrO2 SPEs at different pHs. (A) in bulk solution of B-R buffer or using microfluidic paper (B) in B-R buffers saturated with air or N2 (C) in different buffer systems. Please click here to view a larger version of this figure.
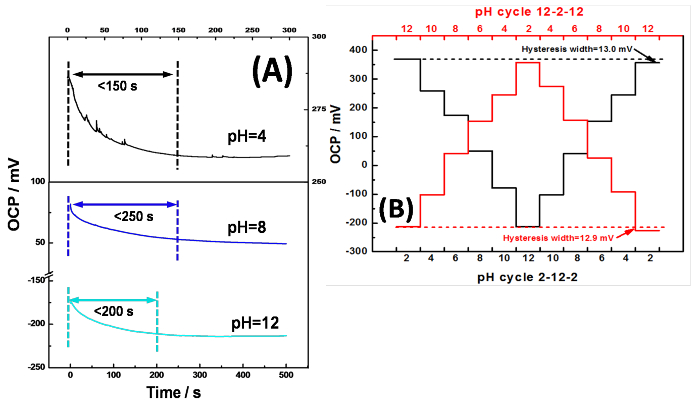
Figure 4: (A) Potential-time curves of RGO-IrO2 SPEs at pH 4, 8 and 10 in B-R buffer. (B) Hysteresis widths of RGO-IrO2 SPEs in B-R buffers at different pH with loop cycles of 2-12-2 and 12-2-12. Please click here to view a larger version of this figure.
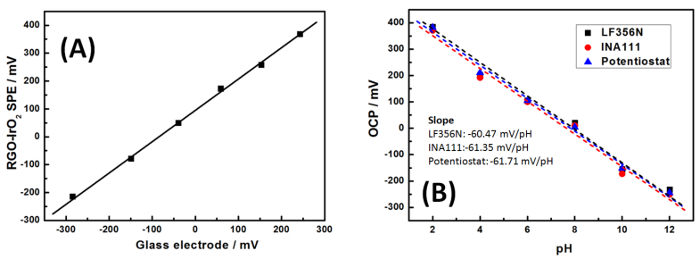
Figure 5: pH responses of RGO-IrO2 SPEs in B-R buffer at different pHs. (A) correlation with a standard commercial pH meter with glass electrode (B) comparison of the measurements by different devices. Please click here to view a larger version of this figure.
Discussion
Device Setup
The pH sensor works by measuring the OCP between the working and reference electrodes, since it changes proportionally to the negative logarithm of H+ concentration. The measurements can be achieved both by a lab-based potentiostat such as CHI 660D and simple pH meter constructed on breadboard with reading by multimeter. Two different portable pH meters were built similarly on breadboards using two 9 V alkaline batteries, a digital multimeter, as-synthesized IrO2-RGO-SPE and different OpAmps, which is two serial LF356N or one INA111. The entire cost of such pH meters is typically under $25 including around $1 apiece of disposable pH IrO2-RGO-SPEs costing (cost and time to conduct chemical preparation and electrochemical depositions are not included). Figure 1C shows the circuit diagrams and connections with two LF356N or one INA111. Schematic of the entire device according to the connection pins of INA111 is shown in Figure 1D. Two 9 V batteries were used to create a dual supply of +/- 9 V to power the op amps by connecting to the input pins 2 and 3. A 1/4-W 4-band gain resistor (RG) of 10 MΩ (5% tolerance) is connected between pins 1 and 8, resulting in a desired gain (G) close to 1, as calculated from Eq (1). Due to the comparatively low internal resistance of the multimeter, a sufficiently large load resistor is grounded and connected between shorted pins 5 and 6, which further connect the negative and positive probes of multimeters for stable OCP readings. As a result, the output voltage which can be calculated from Eq (2) is exactly equal to the potential difference between IrO2-RGO working electrode and Ag/AgCl reference electrode, corresponding to the solution pH.
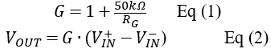
Where G is the gain of INA111, Vout is sensor output (V), and V+in and V–in are voltage at the non-inverting and inverting inputs of OpAmps, respectively.
Surface Morphology
Figure 2A scanning electron microscopy (SEM) image shows the surface morphology of the intermittent RGO film formed with a typical wrinkled texture composed of flexible and ultrathin graphene sheets spreading through the rough screen-printed carbon electrode surface. The GO reduction occurs at potentials more negative than -0.65 V and is rapid, irreversible and controllable without reducing reagents7. After IrO2 deposition, the black RGO film visually and notably turned dark purple, showing the characteristic electrochromic feature of anodically electrodeposited IrO2. IrO2-RGO nanohybrid thin film (Figure 2C) in µm-range thickness is observed with homogeneously dispersed nanoscale crystallites on the entire surface. The film appears to be uniform and smooth, without any noticeable cracks or mud structures. Moreover, an interesting finding in the current study is that IrO2 films cannot form on the screen-printed graphitic carbon substrates without the assistance of RGO under the same conditions (Figure 2B). Also the good film forming ability of graphene is conceived the key component in the thin film deposition process. The oxalates in the deposition solution can trigger complex formation and prevent iridium precipitation in alkaline medium. The deposition is achieved by anodic oxidation of oxalated Ir(IV) compounds with CO2 release and concomitant Ir(IV) oxide formation at the anode surface, as described by Yamanaka8,9
[Ir(COO)2(OH)4]2– ⇔ IrO2 + 2CO2 + 2H2O + 2e–
pH Measurement Performance
A general mechanism of metal oxides in pH sensing has been indicated by Fog and Buck to be associated with the ion-exchange processes within the OH group-bearing surface10. Different IrO2-based electrodes exist in nature due to the differences in their synthesis mechanisms. A number of possible mechanisms are proposed involving pH-dependent redox intercalation equilibrium between two oxidation states of Ir oxides11. As in the case of electrochemically-synthesized Ir oxides, the predominant state is the hydrated form8 which delivers super-Nernstian responses with a sensitivity superior than that of anhydrous forms at 59 mV/pH. The mechanism can be explained using the following equation8,9:
2[IrO2(OH)2 · 2H2O)]2– + 3H+ + 2e– ⇔ 2[IrO2(OH)2 · 2H2O)]2– + 3H2O
As shown in Figure 3A (black), the IrO2-RGO electrode showed a well-defined linear characteristic over a wide pH range from 2 to 12, with a slightly super-Nernstian slope of -61.71 mV/pH which is closer to anodically electrodeposited IrO2 films with near-Nerstian responses11,12 rather than those with super Nernstian13,14. Possibly this happens due to the different electrodeposition conditions and substrates in each case. It is possible that a mixture of anhydrous and hydrated IrO2 film is formed on graphene during the anodic electrodeposition. The negligible difference of measured pH between patterned paper microfluidic device (Figure 3A, red) and bulk solution indicates the fibrous cellulose paper matrix does not impede diffusion of hydrogen ions to any noticeable degree. Dissolved atmospheric oxygen exists ubiquitously in samples can sometimes greatly influence potential readings due to its redox processes, especially in biological systems12. When the electrode is placed in N2– or air-saturated buffer solution, there are only minor differences (Figure 3B). Meanwhile, since the ionic strengths and compositions are different depending on buffers, a number of pH buffers were tested including commercial pH calibration buffer, B-R buffer, phosphate buffered saline (PBS) and NaOH/HCl adjusted DI water (Figure 3C). The sensitivities (mV per unit pH) are almost identical in all buffers. However, an appreciable potential drift was observed in PBS buffer which has a relatively narrower pH range. This may be attributed to different conditional standard potentials (E0' term in the equilibrium Nernst equation) of IrO2-RGO pH electrode in PBS.
Response time, a key factor in any sensing application, is usually defined as the time required reaching certain percentages of equilibrium OCP. Typical response time is less than 250 sec under all pH but could be strongly pH-dependent11. Hysteresis, or the so-called memory effect, is a well-known phenomenon with glass and metal oxide pH electrodes during repetitive uses of the same electrode. This phenomenon of hydrogen ion-selective electrodes is considered as the result of delayed responses to the pH change. The IrO2-RGO pH electrode was tested in pH buffers from low to high, and in turn from high to low. This loop cycles of pH 2-12-2 and 12-2-12 were assessed by successively measuring OCP of different pH buffers in the cycles (Figure 4B). The hysteresis widths are calculated to be around 13 mV in both cycles, which are acceptable and accurate in routine pH measurements, particularly regarding the wide pH range studied here.
To further validate the IrO2-RGO nanohybrid thin film pH sensor, the performance was tested in parallel with a standard glass electrode with a pH/mV/Ion/Conductivity Meter (Figure 5A). The results correlate well each other, suggesting the high accuracy and reliable performance of the developed pH sensor. To truly achieve on-site pH measurements featuring the portable electrochemical paper microfluidic device, two simple digital pH meters were built with slightly different configurations. Measured pH using both meters was highly consistent with the lab-based electrochemical analyzer. Reproducibility was tested multiple times with the same electrode in different pH B-R buffers, and also with different electrodes. The relative standard deviation (RSD) values were typically all <15%.
In conclusion, a rapid, controllable and green electrochemical method is developed for synthesis of uniform and smooth IrO2-RGO nanohybrid thin films on rough SPE surface, assisted by the good film-forming and supporting capabilities of RGO. The resulting solid-state electrode exhibited a slightly super-Nernstian response with a high sensitivity of -62 mV/pH and with good linearity in a wide range of pH from 2 to 12. The electrodes also have small hysteresis, fast response time, reproducibility, and good agreements with commercial glass electrode equipped lab-based pH meter. A miniaturized paper microfluidic pad was fabricated by PDMS hydrophobic barrier patterning. A simple pH meter was fabricated using two 9 V alkaline batteries, a digital multimeter, and OpAmps. Measured pH results from the sensor correlated well with those obtained using lab-based techniques. Thus the sensor which combines advantages of both pH meters and pH strips is a promising platform for future on-site or point-of-care pH measurements, especially under limited-resources status.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant from the Water Equipment and Policy (WEP) NSF Industry/University Cooperative Research Center (I/UCRC). The authors are also thankful to the Hjalmar D. and Janet W. Bruhn Fellowship and Louis and Elsa Thomsen Wisconsin Distinguished Graduate Fellowship provided to J. Y. at UW-Madison
Materials
| Screen-printed electrodes | Zensor | TE100 | 3-electrode integrated |
| acrylonitrile butadiene styrene (ABS) | |||
| Polydimethylsiloxane (PDMS) prepolymer and cross linker mixture | Dow-Corning Co. | Sylgard 184 | 10:1 mixture w/w |
| Whatman No. 1 filter paper | GE Healthcare co. | ||
| 3D milling system | Roland DGA Co. | iModela IM-01 | |
| PDMS stamp and vacuum cover | Roland DGA co. | Sanmodur | Synthetic resin tablet |
| hand-operated vacuum pump | Cole-Parmer co. | ||
| Electrochemical workstation | CH Instruments | CHI 660D | |
| LF356N operational amplifiers | Texas Instruments Inc. | ||
| INA111 high speed field-effect transistor (FET)-input instrumentation amplifier | Burr-Brown Inc. | ||
| DMM914 digital multimeter | Tektronix Inc. | 70979101 | |
| From Fisher or Sigma: | |||
| iridium tetrachloride (IrCl4) | |||
| 50% (w/w) hydrogen peroxide (H2O2) | |||
| oxalic acid dihydrate | |||
| potassium carbonate (K2CO3) | |||
| phosphoric acid | |||
| acetic acid | |||
| boric acid | |||
| sodium hydroxide (NaOH) | |||
| Na2HPO4 | |||
| NaH2PO4 |
References
- Greenblatt, M., Shuk, P. Solid-state humidity sensors. Solid State Ionics. , 995-1000 (1996).
- Nie, Z., Nijhuis, C. A., Gong, J., Chen, X., Kumachev, A., Martinez, A. W., Narovlyansky, M., Whitesides, G. M. Electrochemical sensing in paper-based microfluidic devices. Lab Chip. 10, 477-483 (2010).
- Yang, J., Nam, Y. G., Lee, S. -. K., Kim, C. -. S., Koo, Y. -. M., Chang, W. -. J., Gunasekaran, S. Paper-fluidic electrochemical biosensing platform with enzyme paper and enzymeless electrodes. Sens. Actuators, B. 203, 44-53 (2014).
- Delaney, J. L., Hogan, C. F., Tian, J., Shen, W. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal. Chem. 83, 1300-1306 (2011).
- Lankelma, J., Nie, Z., Carrilho, E., Whitesides, G. M. Paper-based analytical device for electrochemical flow-injection analysis of glucose in urine. Anal. Chem. 84, 4147-4152 (2012).
- Dossi, N., Toniolo, R., Pizzariello, A., Impellizzieri, F., Piccin, E., Bontempelli, G. Pencil-drawn paper supported electrodes as simple electrochemical detectors for paper-based fluidic devices. Electrophoresis. 34, 2085-2091 (2013).
- Yang, J., Gunasekaran, S. Electrochemically reduced graphene oxide sheets for use in high performance supercapacitors. Carbon. 51, 36-44 (2013).
- Yamanaka, K. Anodically electrodeposited iridium oxide films (AEIROF) from Alkaline Solutions for Electrochromic Display Devices. Jpn. J. Appl. Phys. 28, 632-637 (1989).
- Yamanaka, K. The electrochemical behavior of anodically electrodeposited iridium oxide films and the reliability of transmittance variable cells. Jpn. J. Appl. Phys. 30, 1285-1289 (1991).
- Fog, A., Buck, R. P. Electronic semiconducting oxides as pH sensors. Sens. & Act. 5, 137-146 (1984).
- Bezbaruah, A. N., Zhang, T. C. Fabrication of anodically electrodeposited iridium oxide film pH microelectrodes for microenvironmental studies. Anal. Chem. 74, 5726-5733 (2002).
- Marzouk, S. A. M., Ufer, S., Buck, R. P., Johnson, T. A., Dunlap, L. A., Cascio, W. E. Electrodeposited iridium oxide pH electrode for measurement of extracellular myocardial acidosis during acute ischemia. Anal. Chem. 70, 5054-5061 (1998).
- Prats-Alfonso, E., Abad, L., Casañ-Pastor, N., Gonzalo-Ruiz, J., Baldrich, E. Iridium oxide pH sensor for biomedical applications. Case urea-urease in real urine samples. Biosens. Bioelectron. 39, 163-169 (2013).
- Bitziou, E., O’Hare, D., Patel, B. A. Simultaneous detection of pH changes and histamine release from oxyntic glands in isolated stomach. Anal. Chem. 80, 8733-8740 (2008).

