Characterizing Electron Transport through Living Biofilms
Summary
A protocol for measuring electrical conductivity of living microbial biofilms under physiologically relevant conditions is presented.
Abstract
Here we demonstrate the method of electrochemical gating used to characterize electrical conductivity of electrode-grown microbial biofilms under physiologically relevant conditions.1 These measurements are performed on living biofilms in aqueous medium using source and drain electrodes patterned on a glass surface in a specialized configuration referred to as an interdigitated electrode (IDA) array. A biofilm is grown that extends across the gap connecting the source and drain. Potentials are applied to the electrodes (ES and ED) generating a source-drain current (ISD) through the biofilm between the electrodes. The dependency of electrical conductivity on gate potential (the average of the source and drain potentials, EG = [ED + ES]/2) is determined by systematically changing the gate potential and measuring the resulting source-drain current. The dependency of conductivity on gate potential provides mechanistic information about the extracellular electron transport process underlying the electrical conductivity of the specific biofilm under investigation. The electrochemical gating measurement method described here is based directly on that used by M. S. Wrighton2,3 and colleagues and R. W. Murray4,5,6 and colleagues in the 1980's to investigate thin film conductive polymers.
Introduction
Extracellular electron transport (EET) is a process that enables certain microorganisms to transport electrons between intracellular metabolic processes and insoluble electron acceptors or donors that reside outside the cell, ranging from natural minerals to electrodes. In some cases, EET enables microorganisms to form electrically conductive multi-cell thick biofilms on electrode surfaces, in which cells not in direct contact with the electrode can still utilize it as a metabolic electron acceptor or donor. There is considerable interest in such biofilms as electrode catalysts for various applications, such as microbial electrosynthesis, contaminant sensing/removal, and remote energy generation and storage,7,8,9,10,11,12,13,14 due to the diversity of metabolic processes performed by microorganisms and the durability of microbial biofilms compared to enzyme-based bioelectrodes.15,16 In addition, EET pathways may potentially be utilized to electrically control or signal changes in naturally occurring or genetically engineered microbial metabolic processes involved, for example, in production of a desired product or detection of a target analyte or stimulus. The electrical conductivity of electrocatalytic biofilms, which sets them apart from other biological materials, is a central aspect of their electrocatalytic properties, yet little is understood about the underlying EET process in the electrode environment, and that which is known is highly contested.17,18,19,20,21,22,23,24
Described here is a 2-electrode method to measure conductivity through living, electrode-grown biofilms using interdigitated electrode arrays (IDAs). IDAs consist of parallel rectangular electrodes patterned on flat glass surface such that every other band is connected at opposite sides of the array resulting in 2 electrodes (the source and drain). Careful examination of an IDA (see for example, Figure 6.12b of ref #1) reveals that that the gaps separating adjacent bands are also connected in such a way as to form a single gap that weaves back and forth across the array separating the two electrodes. The result is a long and narrow gap separating the source and drain electrodes, yielding very high source-drain currents when a conductive material is formed, cast, polymerized, or grown (in the case of the type of biofilms considered here) over the array. In addition, the small size of the electrodes results in small background current due to capacitance charging and to change in oxidation state of the conductive material with change in gate potential, since the amount of material needed to make conductivity measurements using IDAs is so small. The technique of IDA-based electrochemical gating described here, developed to characterize thin film conductive polymers,2,3,4,25 has only recently been applied to living systems.18 Another technique used to measure conductivity of living biofilms utilized a large format split source and drain electrodes and source meters to set the gate potential.26,27 However, concerns over these methods have been detailed previously.18
The protocol below encapsulates our experience with making conductivity measurements of living Geobacter sulfurreducens and biocathode MCL biofilms. G. sulfurreducens is a model electrode reducing organism able to use insoluble materials, including electrodes, as the sole metabolic electron acceptor. Additionally, it forms thick biofilms that are able to transport electrons over multiple cell lengths, making it an ideal model organism to study anodic long-distance extracellular electron transfer. We also include details for the study of biocathode MCL, an aerobic, autotrophic mixed community biofilm isolated from the cathode of a benthic microbial fuel cell. Biocathode MCL (named for the three primary constituents – Marinobacter, Chromatiaceaea and Labrenzia) is capable of oxidizing an electrode as its sole electron donor and transporting electrons over multiple cell lengths, making it an interesting cathodic system to study. Additionally, biocathode MCL has the highest reported conductivity for a living system to date using these methods. The inclusion of these diverse electroactive biofilms in this protocol is meant to highlight that this technique is applicable to measure the transport of electrons through any living biofilm able to electrically interact with electrodes.
Protocol
1. Interdigitated microelectrode array (IDA) preparation
- Obtain commercially available IDA electrodes patterned on a nonconductive substrate or synthesize them using standard lithographic methods.28
NOTE: IDA dimensions and/or materials can be varied based on desired conditions for different experiments. IDAs used here were obtained commercially and consisted of two interdigitated gold microelectrodes patterned on a glass substrate connected to large electrode pads at opposite ends of the array. The electrodes are exposed while the bus lines connecting the electrodes to the large contact pads are coated with thin insulating material. IDAs used here are comprised of two sets of 10 µm-wide and 2 mm long gold microelectrode bands (source and drain) spaced 5 µm apart patterned on a flat glass surface. IDAs used here had 65 electrode pairs (130 total interdigitated bands). Alternating bands are connected to electrode pads at opposite ends of the array. - Wire and insulate IDA
- Attach a wire to each large electrode pad using conductive silver epoxy.
- Prepare conductive epoxy according to manufacturer's instructions for the specific epoxy in use with a mixing rod or pipette tip (instructions may vary by manufacturer).
- Place a wire on each gold pad, secure in place using lab tape. Cover the wire and pad with silver epoxy using a mixing rod or pipette tip.
- Carefully move to an 80 °C oven for 1 h to cure or cure based on manufacturer's recommendations for the specific conductive silver epoxy used.
- After the epoxy cures, use a multimeter to ensure electrical connectivity between the end of the wire and the pads. The resistance between the wire and the pad should be < 5 Ω. Also, use the multimeter to verify that no conductive epoxy is connecting multiple electrode pads, as this can short the array. If conductive epoxy is connecting multiple leads, use a scribe to isolate the leads.
- Insulate the epoxied connection by coating the connection with a thermal, electrical, and waterproof insulating material. Flame retardant polyurethane resins are often suitable.
- Remove the tip of a 15-mL conical centrifuge tube (at approximately the 2.5 mL mark) as a mold for the insulating material. Make two small holes in the bottom for the wires to protrude through using a 21g needle.
- Insert the IDA into the mold and wires through the holes in the bottom of the mold.
- Prepare the specific insulating material. Follow instructions provided with the specific resin obtained.
- Pipette the insulator into the mold so that the silver epoxy is completely covered (~2.5 mL) and allow to dry according to manufacturer's specifications.
- Attach a wire to each large electrode pad using conductive silver epoxy.
2. Electrochemical reactor setup, testing, and inoculation
- Set up the electrochemical cell.
- Insert IDA, counter electrode, and reference electrode into the electrochemical cell.
NOTE: The electrochemical reactor used can vary widely, as long as all of the electrodes fit inside. One consideration is that the reference electrode should be as close as possible to the working electrode given the practical limits of the reactor. Here, we use a single chamber reactor with the reference electrode ~2-3 cm from the working electrodes. Also, the counter electrode should be larger than the working electrode to ensure that it is not limiting in the system. - If working with a pure culture, sterilize the reactor with the counter electrode inside. Sterilize the reference electrode by soaking in bleach for 10 min and sterile deionized water for 10 min. Sterilize the IDA by dipping in bleach for 5 s followed by sterile deionized water for 10 s before insertion into the reactor.
- Fill the electrochemical cell with sterile medium suitable for biofilm growth. For G. sulfurreducens17,18, use freshwater medium excluding fumarate. For biocathode MCL,9 use artificial seawater medium.9
- Connect the electrodes to a bipotentiostat. Connect one IDA electrode to the working lead 1, the other IDA electrode to the working lead 2, the reference electrode to the reference lead, and the counter electrode to the counter lead.
- Insert IDA, counter electrode, and reference electrode into the electrochemical cell.
- Electrochemical testing of IDAs.
NOTE: The major goal of this test is to make sure that the two electrodes are electrically isolated. All electrochemical techniques are available in the software used to control the bipotentiostat.- Perform control conductivity tests (before biofilm growth) to ensure proper IDA function using a bipotentiostat.
- Measure the open circuit potential of each electrode for 1 min.
NOTE: On some instruments, open circuit potential must be achieved by using the galvanostatic collection program and setting the current to 0 mA. - Measure the open circuit potential of electrode 2 while performing cyclic voltammetry on electrode 1 between +0.2 V to +0.6 V (vs. SHE) at 20 mV/s.
NOTE: Other bounds and scan rates for CV can be used if desired. However, avoid potentials that will result in hydrogen or oxygen generation. - Verify that the open circuit potential measured at electrode 2 does not mirror the potential of electrode 1 during the CV using the potentiostat software.
NOTE: The open circuit potential of electrode 2 may change, but it should be independent of the potential of electrode 1. - Repeat steps 2.2.1.1 through 2.2.1.3 except control the potential of electrode 2 and measure the open circuit potential of electrode 1.
- Measure the open circuit potential of each electrode for 1 min.
- Set the potential of working electrodes to the desired growth potential of the relevant electroactive biofilm using the bipotentiostat software. For example, use +0.5 V (vs. SHE) for Geobacter sulfurreducens or +0.31 V (vs. SHE) for biocathode MCL.
- Perform control conductivity tests (before biofilm growth) to ensure proper IDA function using a bipotentiostat.
- Grow relevant electroactive biofilm
- Inoculate the electrochemical reactor from a stock culture/enrichment of the desired electrochemically active microorganisms using standard aseptic microbiological techniques. For standard tests, inoculate in a 1:20 ratio (inoculum to reactor volume) of an OD600 = 0.5 culture.
- Set the stirring in the reactor to the desired level (~200 rpm) and the incubator/ water bath to the desired temperature based on the growth conditions of the biofilm of interest. For G. sulfurreducens, use 30 °C for optimal growth.
- Incubate the system based on specific requirements of the microorganism(s) of interest until the biofilm bridges the gap separating the two electrodes. For stationary G. sulfurreducens biofilm, incubate for ~7-10 days. For biocathode MCL, incubate ~7 days. In each case, control the temperature at 30 °C.
3. Electrochemical gating experiments
- Select the experimental parameters that will be used to determine the current-potential dependence for the gating measurements.
- Determine the range of gate potentials that will be applied to the IDA to obtain the conducted current (ISD) versus gate potential (EG) curve for the system.
NOTE: The range of gate potentials examined should cover all potentials with possible redox activity. If no information about the system of interest is available, a wide potential range should be used (-0.55 to +0.6 V vs. SHE). A trial and error approach can be used to fine tune the range based on the system under study. Gate potential is defined as:
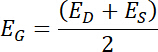
where ED is the potential of the drain electrode and ES is the potential of the source electrode. The range of gate potentials used is constrained by the requirements and limitations of the specific system of interest.18
NOTE: Source and drain potentials that will result in oxygen and hydrogen evolution should be avoided as these processes can damage the biofilm. - Determine the source-drain voltage (VSD) that will be used as the driving force for electron transport through the film from the source to the drain. Source-drain voltage is defined as:
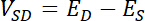
NOTE: The source-drain voltage should be sufficiently small so that the ISD scales linearly with VSD.17 - Choose a scan rate (v) at which EG is changed linearly with time that is independent of ISD so that the system can be approximated to be at steady state for each gate potential.
NOTE: A scan rate of less than 0.002 V/s is often used for biological systems.29,30 - Set up the bipotentiostat software to perform the gating measurements (i.e. sweep the gate potential) over the selected range, at the selected source-drain voltage, and at the desired scan rate based on the above considerations.
NOTE: For previous gating measurements using G. sulfurreducens,17,19 EG = -0.55 V to 0.6 V (vs. SHE), VSD = 0.01 V or 0.1 V, v = 0.001 V/s. For Biocathode MCL, EG = 0.25 V to 0.7 V (vs. SHE), VSD = 0.002 V, v = 0.0002 V/s.- Additionally, perform a baseline measurement with VSD = 0.000 V (i.e., a CV at each individual electrode taken simultaneously) at the same v chosen in Step 3.1.3.
- Perform gating measurements using the conditions in 3.1.4 under both turnover (with soluble electron donor or acceptor present) and non-turnover (without soluble electron donor or acceptor) conditions.
NOTE: Non-turnover conditions are advantageous because measurements are not obscured by the oxidation or reduction of soluble compounds for cellular metabolism, although similar results should be obtained regardless of which condition is used after subtracting background currents (detailed in 3.1.8).- Achieve turnover conditions by using the same reactor medium as used for bacterial growth on the electrode. This medium contains a soluble electron donor, such as acetate for G. sulfurreducens,17 or acceptor, such as oxygen for Biocathode MCL.
- Achieve non-turnover conditions by making the same reactor medium used for bacterial growth on the electrode, except omit the soluble electron donor or acceptor. After ensuring that the potentiostat is off, remove the medium aseptically and add in fresh medium without the electron donor for anodic systems or acceptor for cathodic systems. Alternatively, a continuous flow system can be set up to slowly replace the medium with the desired medium for nonturnover conditions.
NOTE: If oxygen is the soluble electron acceptor (as for biocathode MCL), sparge the system with a mixture of ~15% CO2 and 85% N2 (or a gas mixture that will maintain the correct pH in the medium).
- After completion of the gating measurements, use the potentiostat software to set the potential of each electrode back to the growth potential to allow the system to re-stabilize (using the same values as in 2.2.2).
- If all the conditions described above are met (ISD is independent of v and scales linearly with VSD), convert ISD values to conductivity using the following equations as previously described31
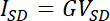
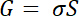
- where G is the conductance and S is a scaling factor that is system dependent, and factors in variables such as electrode size, gap size, and biofilm height. For certain systems, S can be determined from predetermined equations.31 Alternatively, S can be calculated numerically using a modeling software, as detailed previously.17
- Subtract the background currents to identify the shape and magnitude of the conducted current. Either subtract the current generated at VSD = 0.00 V from the current generated at VSD = 0.01 V or subtract the drain current from the source current generated with a VSD = 0.01 V. Either method removes background currents, leaving only the conducted current.
- Determine the range of gate potentials that will be applied to the IDA to obtain the conducted current (ISD) versus gate potential (EG) curve for the system.
- Temperature dependent electrochemical gating measurements
- Determine the range of temperatures of interest. This range is highly dependent on the system under study, however physiologically relevant temperatures should be used.
NOTE: Previous studies have used a temperature range of 10 °C to 30 °C to study electron transport under physiologically relevant conditions for mesophilic microorganisms.17 - Obtain a recirculating water bath or incubator to regulate reactor temperature and a control reactor to ensure that the set point and actual temperature of the medium is the same.
- Place a thermometer or thermocouple in a control reactor where the IDA would be.
- Make ISD measurements (see 3.1.4) over the range of temperatures selected under turnover and nonturnover (described in Step 3.1.5) conditions using the bipotentiostat following one of the two procedures described below.
- Generate ISD vs. EG curves, as described above (see 3.1), for each temperature over the temperature range of interest. For materials that exhibit redox conductivity, such as G. sulfurreducens and Biocathode MCL, the peak current from each temperature is used to determine the ISD vs. T dependence.
NOTE: This method is used to generate a full ISD vs. EG curve for each temperature, but requires more time than the second option. - Alternately, set and hold the IDA at the gate potential that yields maximum conducted current using the bipotentiostat (obtained from the ISD vs EG curve, Step 3.1.4) and record the maximum conducted current using the bipotentiostat while the temperature is cycled between the range of temperatures selected using the onboard controls of the water bath or incubator.
NOTE: With the electrode/reactor geometry used here, the ISD and T are allowed to stabilize for at least 20 min and an average stable ISD is used for each temperature. More or less stabilization time may be required based on the specific system. This method is faster than the first and causes less stress to the biofilm. However, full gating curves are not generated. - Cycle the temperature from one set point to the other and back again using the onboard controls of the incubator or the water bath to determine the reversibility of the reaction to ensure that the temperature cycling does not harm the biofilm.
- Generate ISD vs. EG curves, as described above (see 3.1), for each temperature over the temperature range of interest. For materials that exhibit redox conductivity, such as G. sulfurreducens and Biocathode MCL, the peak current from each temperature is used to determine the ISD vs. T dependence.
- Reset the temperature back to the normal growth temperature using the onboard controls of the incubator or water bath and allow the system to stabilize.
NOTE: For a redox conductor, the ISD versus 1/T data can be fit with the Arrhenius rate expression, which allows the calculation of activation energy as follows:
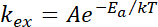
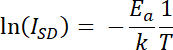
where Ea is the activation energy for electron transfer between adjacent redox cofactors and k is the Boltzmann constant.
- Determine the range of temperatures of interest. This range is highly dependent on the system under study, however physiologically relevant temperatures should be used.
Representative Results
IDAs were wired, insulated and tested to ensure that the two electrodes were electrically isolated from each other (Figure 1). Reactors were assembled, inoculated with G. sulfurreducens, and incubated until a biofilm bridged the gap between the electrodes. The G. sulfurreducens biofilm can be visually seen to be covering the array. Other biofilms may require the researcher to do an electrochemical gating measurements to see if the two electrodes have been electrically connected. Microscopy should also be used to verify connection between the electrodes of the array. Electrochemical gating experiments were carried out to determine the dependence of ISD on EG (Figure 2). The conductivity of the living film is then calculated using the conducted current measured in the gating experiments. The precision and accuracy of these measurements was high due to the high signal to noise ratio possible with the IDA configuration. The temperature dependence of ISD on T was also determined along with an activation energy for electron transport through the biofilm (Figure 2). The results obtained here are similar to those previously observed17,18 and support the hypothesis that G. sulfurreducens and biocathode MCL biofilms behave similarly to redox conductors where electrons are transferred through the biofilm by hopping between redox cofactors close in proximity.
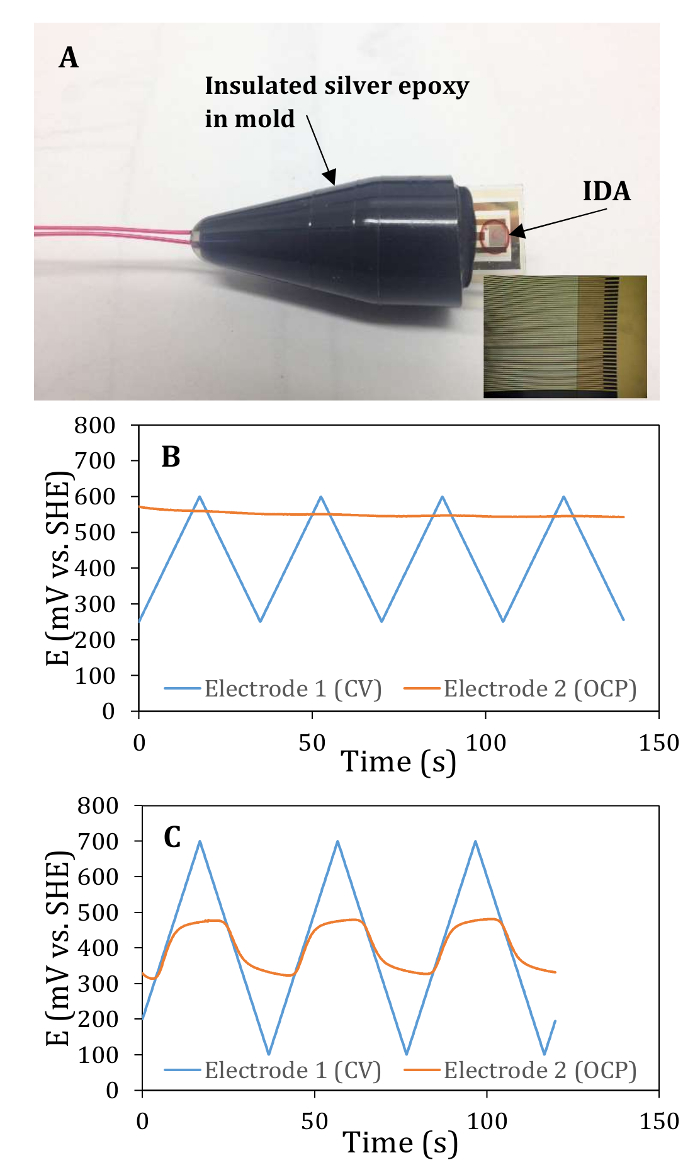
Figure 1: IDA set up and control electrochemical tests. (A) An IDA that has been wired and insulated. Inset: Enlarged imaged of the array showing the interdigitated electrodes and one of the large electrode pads. Separate counter and reference electrodes are placed into the electrochemical cell along with the IDA to perform experiments. (B) Electrochemical control tests exhibiting electrical independence of each electrode. The open circuit potential of electrode 2 does not respond to the changing potential of electrode 1 during CV, indicating that the electrodes are not shorted and can be used for biofilm gating measurements. (C) Same as B, except the potential of electrode 2 does shift during CV of electrode 1, indicating that the electrodes are shorted and should not be used for gating measurements. This IDA was not used in further experiments. Please click here to view a larger version of this figure.
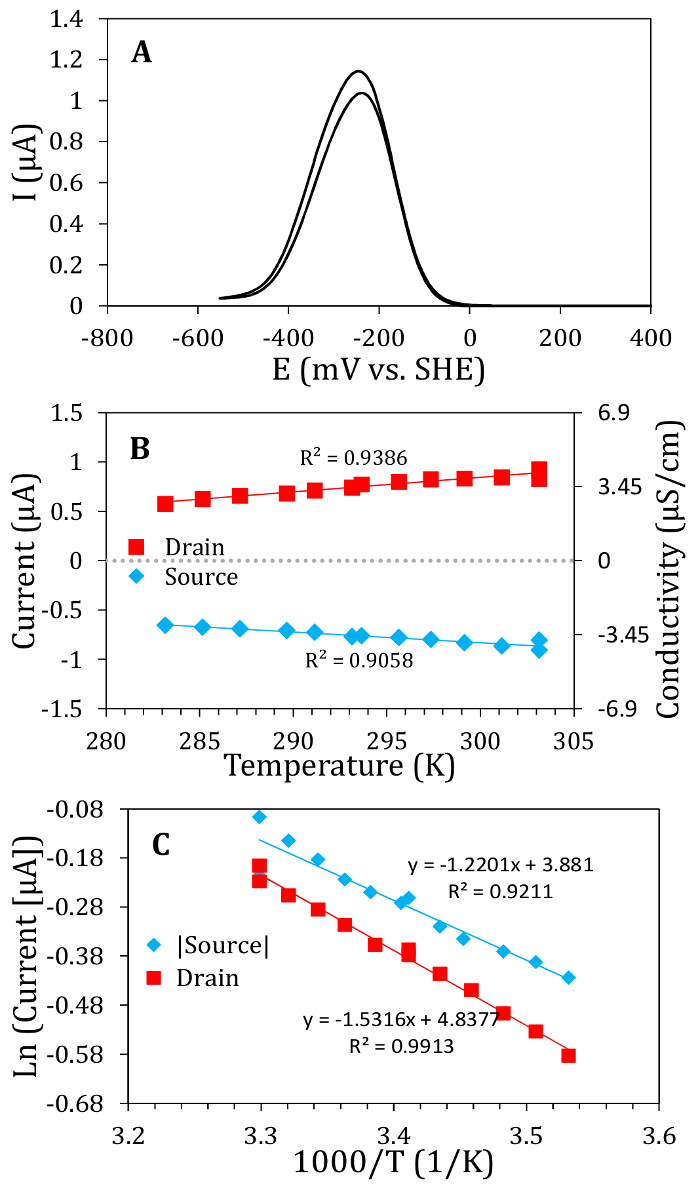
Figure 2: Electrochemical gating experiments. (A) Electrochemical gating measurements of a living, electrode grown G. sulfurreducens biofilm. The peak shaped ISD-EG curve is indicative of incoherent, multi-step electron transport through the biofilm. The conducted current curve was obtained by subtracting the source from the drain current (and dividing by 2) obtained at each gate potential to eliminate background currents. For examples of raw current data taken with VSD = 0 and VSD = 0.01 V, the reader is referred to the Supporting Information of previous work.18 (B) Temperature dependent gating measurementsover a physiologically relevant range exhibiting an increase in the conductivity as the temperature is increased, a property observed for redox conductors.4 (C) Transformation of the ISD – T data and fit to the Arrhenius equation. The linear fit to the Arrhenius equation is indicative of a multi-step electron transfer process. The activation energy for electron transport through a G. sulfurreducens biofilm is calculated from the slope of the curve to be ~0.01 eV, which is consistent with electron transport between redox centers of adjacent c-type cytochromes.32,33,34 Please click here to view a larger version of this figure.
Discussion
During the setup of the IDA, it is critical to test that the source and the drain are not shorted together prior to electrochemical gating measurements, as this will alter the ISD vs. EG curve and could lead to erroneous results and interpretations. It is also critical to select VSD and v such that the current is linearly dependent on VSD and independent of v. If this is not the case, then the equations described above cannot be utilized to calculate conductivity.
At least two background currents must be considered and removed from conducted current measurements. The first is background current due to Faradaic charging/discharging of the redox cofactors as the gate potential is swept. This background current is greatly affected by the amount of redox cofactors that are electrically accessible connected to the electrode surface. A second background current is double layer capacitance. A third background current is due to turnover of metabolic electron acceptors/donors by cells. This background current is only applicable under turnover conditions. Background currents in this study were eliminated by subtracting the source current from the drain current obtained at VSD = 0.01 V. This method assumes that background currents are equal at both electrodes and source-drain currents are equal in magnitude at both electrodes, but opposite in sign. In this case subtracting the source and drain currents yields a conducted current double in magnitude and should be divided by two. It should be noted that this assumption only holds true in the limit of a small VSD, which is system dependent (for G. sulfurreducens, VSD< 0.05 V). Larger VSD values often results in disparate conditions on each electrode and prevents this method of background subtraction from being used. Alternatively, background currents can be removed by subtracting source and drain currents obtained at VSD = 0.0 V from those obtained at VSD = 0.01 V. This method does not assume that the baseline currents of each electrode are the same.
The technique described here is flexible. Most of the parameters described in the protocol are dependent on the system under study and can be altered. For example, the material and dimensions of the IDA can be varied, the temperature range, and range of gate potentials, among other parameters, can be altered to fit the needs of the specific study. Further, standard microbiological and electrochemical techniques are adapted and utilized, making this protocol suitable for researchers from a variety of fields of study.
Here we have described a protocol for studying electron transport in living, electrode grown, electroactive biofilms using IDAs. IDAs have been used previously to characterize electron transport in thin film conducting polymers and can be fabricated using a variety of standard electrode materials and photolithographic techniques.2 The primary advantage of IDAs is the high signal to noise ratio due to i) the long serpentine gap that separates the alternating source and drain electrode bands and ii) the relatively small total electrode surface area compared to the gap size. The electrode geometry is important to consider in gating measurements because the electrode and gap dimensions have a large effect on the signal to noise ratio and therefore on the accuracy of the conductivity measurements made.18
Electrochemical gating experiments of living, electrode-grown G. sulfurreducens biofilms exhibit a clear peak shaped dependence of ISD on EG, suggesting that electrons are transported through the biofilm via incoherent, multi-step hopping, as in redox conductive polymers.4,35 The peak conductivity of the G. sulfurreducens biofilm was found to be ~4 µS/cm, in agreement with previous results generated under similar conditions.17 Further, the gate potential for peak conductivity is similar to the midpoint potential observed for G. sulfurreducens biofilms during turnover CV.17 This has also been observed previously and is postulated to mean that the same electron carriers used by the cells for transporting electrons resulting from acetate metabolism are also used to carry charge from the source electrode to the drain electrode through the biofilm. Other dependences of ISD on EG, such as have been observed in different materials and suggest a different mechanism of electron transfer. For example, the ISD vs. EG curve of the polymer poly(methylthiophene) shows an s-shaped curve and suggests metallic-like electron conduction.36,37
The temperature dependence of conducted current is a critical parameter in determining the mechanism of electron transport through conductive materials. Until recently, only ex-situ samples had been used to investigate the temperature dependence of conducted current through a biofilm.22 Recent results presented here and elsewhere17 obtained a different ISD – T dependence using gating measurements and therefore predict a multi-step, incoherent hopping mechanism of electron transport through G. sulfurreducens biofilms, which is different than a previously proposed mechanism.22
The major limitation of this technique and other similar geometries when evaluating electron transport through a microbial biofilm is that the charge moves laterally between the source and drain electrodes placed in the same plane on a flat surface. The natural flow of electrons through the biofilm, however, is perpendicular to the electrode surface. Using this technique and model, we approximate the biofilm as a homogenous film and interrogate electron flow through only a portion of the biofilm. Experimental validation of the spatial heterogeneity of the biofilm is still necessary to further validate this technique. However, as described above, this method enables in situ measurements with the highest signal to noise ratio available to date. This technique can be used to study charge transport of any material that is able interact with an electrode.
Disclosures
The authors have nothing to disclose.
Acknowledgements
M.D.Y, S.M.G-S., and L.M.T. acknowledge the Office of Naval Research (Award #N0001415WX01038 and N0001415WX00195), the Naval Research Laboratory, and the Naval Research Laboratory Nanosciences Institute; M.Y.E.-N. is supported by the U.S. Department of Energy Grant DE-FG02-13ER16415.
Materials
| IDAs | CH Instruments | 012125 | Manufactured by ALS-Japan; sold by CH Instruments |
| Wire | Digikey | W7-ND | |
| Conductive silver epoxy | Electron microscopy sciences | 12670-EE | |
| Insulating material | 3M | 2131-B | Scotchast flame retardant compound |
| 15 mL conical centrifuge tube | VWR | 89004-368 | |
| 21g needle | VWR | BD-305165 | |
| 5 mL pipette tips | VWR | 82018-842 | |
| 5 mL pipettor | VWR | 89079-976 | |
| Freshwater medium components | Sigma Aldrich | All standard laboratory chemicals | |
| Ammonium chloride | |||
| Sodium phosphate monobasic | |||
| Sodium bicarbonate | |||
| Artificial seawater medium components | Sigma Aldrich | All standard laboratory chemicals | |
| Sodium chloride | |||
| Magnesium chloride hexahydrate | |||
| Magnesium sulfate heptahydrate | |||
| Potassium chloride | |||
| Sodium bicarbonate | |||
| Calcium chloride dihydrate | |||
| Ammonium chloride | |||
| Potassium phosphate dibasic | |||
| Ag/AgCl reference electrode | Basi | MF-2079 | |
| Graphite rod counter electrode | Electron microscopy sciences | 70230 | |
| Recirculating water bath | Thermo Scientific | 152-5256 | |
| Bipotentiostat | Pine Instruments | WD-20 | http://www.voltammetry.net/pine/aftermath/user |
| Stir bars | VWR | 58947-114 | |
| G. sulfurreducens culture | ATCC | 51573 | |
| Jacketed reactor | Pine Instruments | RRPG085 |
References
- Boyd, D. A., et al. . Biofilms in Bioelectrochemical Systems. , 177-210 (2015).
- Natan, M. J., Wrighton, M. S. Chemically modified microelectrode arrays. Prog Inorg Chem. 7, 391-494 (1990).
- Paul, E. W., Ricco, A. J., Wrighton, M. S. Resistance of polyaniline films as a function of electrochemical potential and the fabrication of polyaniline-based microelectronic devices. J Phys Chem-US. 89, 1441-1447 (1985).
- Dalton, E. F., et al. Charge transport in electroactive polymers consisting of fixed molecular redox sites. Chem Phys. 141, 143-157 (1990).
- Chidsey, C. E. D., Murray, R. W. Electroactive Polymers and Macromolecular Electronics. Science. 231, 25-31 (1986).
- Chidsey, C. E. D., Murray, R. W. Redox capacity and direct current electron conductivity in electroactive materials. J Phys Chem-US. 90, 1479-1484 (1986).
- Gregoire, K. P., Glaven, S. M., Hervey, J., Lin, B., Tender, L. M. Enrichment of a High-Current Density Denitrifying Microbial Biocathode. J Electrochem Soc. 161, H3049-H3057 (2014).
- Siegert, M., Yates, M. D., Spormann, A. M., Logan, B. E. Methanobacterium dominates biocathodic Archaeal communities in methanogenic microbial electrolysis cells. ACS Sus Chem Eng. 3, 1668-1676 (2015).
- Wang, Z., et al. A previously uncharacterized, nonphotosynthetic member of the Chromatiaceae is the primary CO2-fixing constituent in a self-regenerating biocathode. Appl Environ Microbiol. 81, 699-712 (2015).
- Marshall, C. W., Ross, D. E., Fichot, E. B., Norman, R. S., May, H. D. Long-term Operation of Microbial Electrosynthesis Systems Improves Acetate Production by Autotrophic Microbiomes. Environ Sci Technol. 47, 6023-6029 (2013).
- Strik, D. P. B. T. B., Picot, M., Buisman, C. J. N., Barrière, F. pH and Temperature Determine Performance of Oxygen Reducing Biocathodes. Electroanalysis. 25, 652-655 (2013).
- Strycharz, S. M., et al. Reductive dechlorination of 2-chlorophenol by Anaeromyxobacter dehalogenans with an electrode serving as the electron donor. Environ Microbiol Report. 2, 289-294 (2010).
- Yates, M. D., et al. Microbial Electrochemical Energy Storage and Recovery in a Combined Electrotrophic and Electrogenic Biofilm. Environ Sci Technol Lett. 4, 374-379 (2017).
- Tender, L. M., et al. Harnessing microbially generated power on the seafloor. Nature Biotechnology. 20, 821-825 (2002).
- Yates, M. D., Siegert, M., Logan, B. E. Hydrogen evolution catalyzed by viable and non-viable cells on biocathodes. Int J Hydrogen Energ. 39, 16841-16851 (2014).
- Fokina, O., Eipper, J., Winandy, L., Kerzenmacher, S., Fischer, R. Improving the performance of a biofuel cell cathode with laccase-containing culture supernatant from Pycnoporus sanguineus. Bioresource Technol. 175, 445-453 (2015).
- Yates, M. D., et al. Thermally activated long range electron transport in living biofilms. Phys Chem Chem Phys. 17, 32564-32570 (2015).
- Yates, M. D., et al. Measuring conductivity of living Geobacter sulfurreducens biofilms. Nat Nano. 11, 910-913 (2016).
- Snider, R. M., Strycharz-Glaven, S. M., Tsoi, S. D., Erickson, J. S., Tender, L. M. Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven. Proc Natl Acad Sci USA. 109, 15467-15472 (2012).
- Strycharz-Glaven, S. M., Snider, R. M., Guiseppi-Elie, A., Tender, L. M. On the electrical conductivity of microbial nanowires and biofilms. Energ Environ Sci. 4, 4366-4379 (2011).
- Malvankar, N. S., Tuominen, M. T., Lovley, D. R. Comment on “On electrical conductivity of microbial nanowires and biofilms” by S. M. Strycharz-Glaven, R. M. Snider, A. Guiseppi-Elie and L. M. Tender, Energy Environ. Sci., 2011, 4, 4366. Energy Environ. Sci. 5, 6247-6249 (2012).
- Malvankar, N. S., et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat Nanotechnol. 6, 573-579 (2011).
- Strycharz-Glaven, S. M., Tender, L. M. Reply to the ‘Comment on “On electrical conductivity of microbial nanowires and biofilms”‘ by N. S. Malvankar, M. T. Tuominen and D. R. Lovley, Energy Environ. Sci., 2012, 5. Energy Environ. Sci. 5, 6250-6255 (2012).
- Strycharz-Glaven, S. M., et al. Electron Transport through Early Exponential-Phase Anode-Grown Geobacter sulfurreducens Biofilms. Chem Electro Chem. 1, 1957-1965 (2014).
- Chidsey, C. E., Feldman, B. J., Lundgren, C., Murray, R. W. Micrometer-spaced platinum interdigitated array electrode: fabrication, theory, and initial use. Anal Chem. 58, 601-607 (1986).
- Li, C., Lesnik, K. L., Fan, Y., Liu, H. Redox Conductivity of Current-Producing Mixed Species Biofilms. PLOS ONE. 11, e0155247 (2016).
- Malvankar, N. S., et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat Nano. 6, 573-579 (2011).
- Ing, N. L., Nusca, T. D., Hochbaum, A. I. Geobacter sulfurreducens pili support ohmic electronic conduction in aqueous solution. Phys Chem Chem Phys. 19, 21791-21799 (2017).
- Fricke, K., Harnisch, F., Schröder, U. On the use of cyclic voltammetry for the study of anodic electron transfer in microbial fuel cells. Energ Environ Sci. 1, 144-147 (2008).
- Marsili, E., Rollefson, J. B., Baron, D. B., Hozalski, R. M., Bond, D. R. Microbial biofilm voltammetry: direct electrochemical characterization of catalytic electrode-attached biofilms. Appl Environ Microbiol. 74, 7329-7337 (2008).
- Kankare, J., Kupila, E. -. L. In-situ conductance measurement during electropolymerization. J Electroanal Chem. 322, 167-181 (1992).
- Byun, H. S., Pirbadian, S., Nakano, A., Shi, L., El-Naggar, M. Y. Kinetic Monte Carlo Simulations and Molecular Conductance Measurements of the Bacterial Decaheme Cytochrome MtrF. Chem Electro Chem. 1, 1932-1939 (2014).
- El Kasmi, A., Wallace, J. M., Bowden, E. F., Binet, S. M., Linderman, R. J. Controlling interfacial electron-transfer kinetics of cytochrome c with mixed self-assembled monolayers. J Am Chem Soc. 120, 225-226 (1998).
- Bortolotti, C. A., et al. The Reorganization Energy in Cytochrome c is Controlled by the Accessibility of the Heme to the Solvent. J Phys Chem Lett. 2, 1761-1765 (2011).
- Gallaway, J. W., Calabrese Barton, S. A. Kinetics of Redox Polymer-Mediated Enzyme Electrodes. J Am Chem Soc. 130, 8527-8536 (2008).
- Thackeray, J. W., White, H. S., Wrighton, M. S. Poly(3-methylthiophene)-coated electrodes: optical and electrical properties as a function of redox potential and amplification of electrical and chemical signals using poly(3-methylthiophene)-based microelectrochemical transistors. J Phys Chem-US. 89, 5133-5140 (1985).
- Jugnet, Y., Tourillon, G., Duc, T. M. Evidence of Intrinsic Extended π-Bonding Band and Metalliclike Behavior in Undoped and Doped Electropolymerized Poly (3-methylthiophene) Films. Phys Rev Lett. 56, 1862-1865 (1986).

