三维组织工程化排列的星形胶质细胞网络, 以概括发展机制和促进神经系统再生
Summary
我们展示了自组装的发展, 三维束纵向排列星胞和过程内的新型生物材料装箱。这些被设计的 “活脚手架”, 显示微米尺度的直径, 但长度延长厘米, 可作为试验床研究神经发育机制或促进 neuroregeneration 通过引导神经元迁移和/或轴突寻.
Abstract
神经和神经退行性疾病往往导致持久的神经缺损, 由于中枢神经系统 (CNS) 的能力有限, 以取代失去的神经元和再生轴突通路。然而, 在神经系统的发育过程中, 神经元的迁移和轴突的扩展通常发生在其他细胞形成的通路上, 称为 “活支架”。为了模拟这些机制, 并设计一个绕过中枢神经系统抑制环境的策略, 本手稿提出了一种制造组织工程 astrocyte-based “活支架” 的协议。为了创建这些结构, 我们采用了一种新的生物材料装箱方案, 诱导星形胶质细胞组装成稠密的三维双极纵向排列的胞和过程的束。首先, 对中空水凝胶微柱进行了组装, 内腔表面涂有胶原细胞基质。然后将游离的大脑皮质星形胶质细胞传送到圆柱形微柱的腔内, 并在 < 350 µm 的临界内径上自发对准, 并收缩产生由稠密束组成的长纤维状电缆。星形胶质细胞的过程和胶原纤维的测量 97% 细胞的生存能力, 实际上完全由星形胶质细胞、胶质纤维酸性蛋白、波形和巢组成。这些对齐的星形胶质细胞网络被发现为神经元附着和对突起扩展提供了一个可容许的基底。此外, 这些结构保持完整性和对齐时, 提取从水凝胶装箱, 使其适合中枢神经系统植入。这些预制结构模仿自然发生的 glial-based “活体支架”在体内的关键细胞元素。因此, 这些工程化的活体支架可以作为试验床, 用于研究神经发育机制体外或促进 neuroregeneration, 方法是引导神经元迁移和/或轴突寻继中枢神经系统变性后体内.
Introduction
中枢神经系统 (CNS) 的能力有限, 以抵消的损失和/或功能障碍的神经元和轴突通路, 伴随的条件, 如创伤性脑损伤 (脑外伤), 中风, 脊髓损伤 (SCI), 和神经变性疾病1 ,2,3,4,5。中枢神经系统的神经再生仅限于大脑中的有限区域, 从而阻碍了丢失神经元的恢复6,7。此外, 由于缺乏定向引导, 存在的生长抑制剂, 和反应 astrogliosis 在神经组织损伤后的中枢神经系统失去的轴突通路的再生是不够的2,8, 9,10。星形胶质细胞在帮助神经元的离子稳态、神经递质清除、突触形成和神经血管耦合方面具有不同的功能11。然而, 随着对神经组织的轻度损伤, 星形胶质细胞在转变为肥厚状态11时, 可能会发生分子、结构和功能上的变化。为了应对严重的神经, 这些变化导致形成了一个包含迁移反应性星形胶质细胞的半影和一个病变的核心, 包括从破裂的血脑屏障 (BBB), 小胶质细胞漏出的白血球,突, 和成纤维细胞11,12,13。这些反应性星形胶质细胞获得丝状的形态学, 无序的过程, 并表现出增加的表达中间丝蛋白和硫酸软骨素蛋白 (CSPGs), 这阻碍神经再生12。尽管胶质瘢痕最初有助于恢复血脑屏障的完整性, 避免对周围健康组织的炎症反应的传播, 它作为一个物理和生物化学的屏障, 反对轴突再生12,14 ,15,16。例如, 遇到胶质瘢痕的轴突显示球状不良生长锥和发育迟缓的生长12。此外, 损伤后星过程的解体阻碍了再生轴突的扩展17。这些抑制特征的结果表现在经常永久的身体和神经损伤, 病人遭受严重的神经后, 包括创伤和 SCI。
不管在中枢神经系统的功能再生面临的外在挑战, 轴突已经被证明具有内在的再生能力。例如, 与胶质瘢痕接触的不良生长锥的动态性质表明, 这些结局保留了它们扩展12的能力。因此, 人们认为, 轴突再生的主要障碍是后中枢神经系统的抑制环境, 并通过减少胶质瘢痕和/或提供跨越疤痕的再生桥来提供更宽松的环境有利.事实上, 先前的研究表明, 中枢神经系统神经元能够通过利用周围神经移植作为桥梁的病变来延长轴突, 这为轴突再生提供了一个更有利的环境12,18, 19。还采取了其他一些战略来利用这种退化的再生能力。例如, 在各种损伤模型中对细胞生长信号通路的操纵导致轴突再生和胶质瘢痕减少10,20,21。此外, 研究表明, 酶 ABC, 克里夫斯 CSPGs 的大部分糖链的治疗, 减少反应性星形胶质细胞分泌 CSPGs 的抑制作用22。尽管取得了令人鼓舞的结果, 但这些方法并没有提供对生长锥的定向指导, 这可能导致异常再生12, 也不能解释神经元的丢失。细胞方法已经被用来克服胶质瘢痕的影响, 并补充丢失的神经细胞, 特别是神经元。有些组有分化活性星形胶质细胞进入神经细胞, 而另一些则将神经祖细胞移植到中枢神经系统病变, 以重新填充损伤区并促进轴突再生23,24,25. 然而, 仅靠干细胞移植是由于存活率低、集成度差和在受损的组织中保持适度的5。此外, 这些细胞的策略无法恢复长距离轴突, 特别是以控制的方式。因此, 生物材料与其他方法的结合正在探索作为各种神经和祖细胞的运载工具和生长因子26。Biomaterial-based 的方法具有高度的设计控制, 以产生模仿特定的物理, haptotaxic, 和 chemotaxic 提示存在于目标主机组织的三维 (3D) 微环境中的构造,27, 28,29,30,31,32,33,34。这些环境信号的再现是最重要的移植细胞呈现本机样的形态学, 增殖, 迁移和信号, 在其他神经生物学特征29。尽管这些优越的性能, 超越传统的细胞种子生物材料支架的进步需要同时促进定向长距离轴突再生和取代丢失的神经元。
一个有前途的替代方法是基于神经组织工程 “活支架”, 这是不同于其他细胞的方法, 由于存在的活神经细胞与预制细胞, 模拟本地神经和/或发展机制, 以促进有针对性的更换, 重建和再生神经回路4,35。生活支架设计的考虑因素包括神经细胞的表型和来源, 以及由任何伴随的生物材料的组成所决定的机械/物理特性和生物化学信号35。在制作体外后, 这些活体支架可以植入体内, 以呈现细胞黏附分子和趋和神经营养信号, 以积极调节神经细胞迁移和轴突生长, 这取决于状态和再生过程的进展35。胶质细胞可以作为生活支架工程细胞的基础, 因为这些细胞介导各种发育机制在体内。在大脑发育过程中, 新的神经元依赖于由心室区的径向胶质细胞向发育中的皮质板延伸的基底过程, 作为定向迁移的活体支架36,37。此外, 扩展生长锥被证明是自我定位, 通过传感路标细胞诱发的诱人和排斥信号, so-called “先驱” 轴突被建议通过沿 pre-patterned 神经胶质的延伸来达到正确的目标。支架35,38,39。因此, 神经胶质细胞是必要的先驱轴突的指导, 后来作为 axon-based “活支架”, 以指导投影的 “追随者” 轴突。此外, 胶质细胞介导的生长机制已被证明坚持后天, 因为细胞跟随侧迁徙流 (RMS), 从脑室区 (SVZ), 在成年大脑中少数残存的神经再生领域之一, 以导航嗅球 (OB)40。这些细胞在 RMS 中迁移到胶质管内 (图 1A-1), 它由纵向排列的星过程组成, 通过直接的细胞-细胞粘连和局部可溶性因子37,41. 最后, 虽然在哺乳动物中枢神经系统损伤导致中断星过程安排形成了一个神经胶质瘢痕, 物理上阻碍轴突再生17, 许多哺乳体系缺乏形成一个有害的胶质瘢痕。相反, 哺乳种类的胶质细胞维持更有条理的、对齐的图案, 这些模式在受伤区域17、42、43中用作参考线。例如, 在哺乳的 SCI 模型中, 轴突被证明与通过病变的神经胶质桥紧密结合生长, 这表明有组织的胶质支架作为促进轴突再生和功能恢复的基质的重要作用 (图 1A-2)42,44,45. 上述神经特征和发展/再生机制的重述可能会产生一种新的工程 glial-based 活体支架, 可同时驱动未成熟的神经元迁移和轴突寻通过其他允许环境, 从而有可能减轻神经元和轴突道变性与中枢神经系统损伤和疾病相关的影响。
我们的研究小组以前曾设计过多种类型的活体支架, 可通过微组织工程化神经网络 (微 TENNs) 和组织对中枢神经系统和周围神经系统 (三七总皂苷) 的轴突重建和再生。工程神经移植 (雄姿), 分别为27,46,47,48。这两种策略都是基于仿生学的。微 TENNs 是解剖学上的启发结构设计, 以结构和功能取代轴突, 连接不同的神经元群体的大脑。雄姿利用轴突促进轴突再生的发育机制, 以 “从动杆” 轴突生长为例, 以 “先锋” 轴突为目标, 实现有针对性的主机轴突再生35,46,48。我们最近利用了生活支架技术的多功能性, 使用了类似的装箱方案作为微 TENNs, 并寻求启发从 glia-based 机制在整个发展中呈现。在这里, 我们开发的结构组成的星束跨越的胶原管的水凝胶微柱49。这些星活体支架的研制首先是用液体琼脂糖来填充毛细管针总成, 以创建一个中空的圆柱形水凝胶, 外径和内径 (ID) 对应的直径管和针分别。在琼脂糖凝胶和提取的水凝胶微柱从毛细管管, 空心内涂层与 i 型胶原蛋白, 以提供一个环境纵容星形胶质细胞粘附和排列束形成 (图 1B-1)。之后, 腔内的大脑皮质星形胶质细胞从产后大鼠幼崽中分离 (图 1B-2)。与 two-dimensional (2D) 的对准技术, 依赖于电场、micropatterned 槽和细胞外基质 (ECM) 蛋白的应用, 活支架技术中的星形胶质星对准依赖于自组装根据可控变量, 如基底曲率 (列 ID), 细胞密度, 和胶原蛋白浓度50,51,52。星形胶质细胞收缩并重塑胶原蛋白, 并获得与自然支架类似的双极性、纵向排列的形态学观察在体内(图 1B-3)。事实上, 我们正积极地使用这些类似电缆的结构作为物理基底, 用于定向迁移未成熟神经元, 以及通过受损的中枢神经系统的不利环境促进轴突再生, 特别是哺乳动物胶质瘢痕 (图 1C)。本文将介绍星微柱的详细制作方法、相衬和预期细胞的免疫荧光图像, 并对当前的局限性和今后的发展方向进行了全面的讨论。技术.
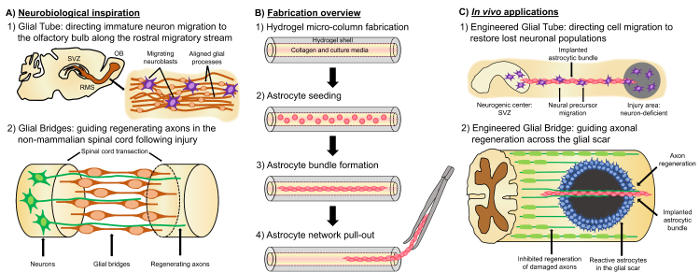
图 1: 对齐的星网络的启发、制造协议和建议的应用程序.(A) 神经生物学灵感: (1) 来源于神经源性脑室区 (SVZ) 的细胞, 利用侧洄游流 (RMS) 中的纵向对中的胶质管向嗅球定向迁移 (OB);(2) 非哺乳动物, 如两栖动物和鱼类可以维持再生后, 神经组织损伤部分由于形成了一个胶质桥连接的两端病变 (如断脊髓), 并作为一个脚手架的指导再生轴突。(B) 制作概述: (1) 微米、空心水凝胶微柱与管腔内涂敷 ECM, (2) 生后大鼠幼崽的初生皮质星形胶质细胞的播种, (3) 自组装的纵向捆绑在文化, 和 (4) 从生物材料装箱的捆绑提取为未来的植入研究。(C)在体内应用程序: (1) 这些活体支架可作为神经源性神经中心定向神经元迁移到重新填充神经元缺陷区域的神经胶质管。(2) non-mammals 的发展机制的重述和神经胶质桥的再生机制, 可以赋予这些星支架的能力, 以引导轴突再生横跨允许哺乳动物胶质瘢痕的环境。请单击此处查看此图的较大版本.
Protocol
Representative Results
Discussion
与三七总皂苷的支持环境相比, 中枢神经系统在处理神经和神经的有害后果方面特别有限。在对哺乳动物中枢神经系统的严重侮辱后, 形成了一个神经胶质瘢痕, 由纤维化和炎症细胞的核心组成, 由一个致密网状的无条理的反应性星形胶质组织所包围, 分泌轴突生长抑制蛋白14。此疤痕作为一个物理和生物化学的障碍, 反对再生轴突结束12。此外, 成年哺乳动物中枢?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
财政支持由国立卫生研究院提供 [U01-NS094340 (卡伦) & F31-NS090746 (Katiyar)], 迈克尔 j ·福克斯基金会 [治疗管道计划 #9998 (卡伦)], 佩恩医学神经科学中心试点奖 (卡伦),国家科学基金会 [研究生研究金 DGE-1321851 (Struzyna)], 退伍军人事务部 [RR & D 值得审查 #B1097 I (卡伦)] 和美国陆军医学研究和装备司令部 [#W81XWH-13-207004 (卡伦) &W81XWH-15-1-0466 (卡伦)]。
Materials
| Acupuncture needle (300 µm diameter) | Lhasa Medical | HS.30×40 | The diameter may be varied according to the desired size for the micro-column lumen. |
| Petri dish | Fisher | 08772B | |
| Dulbecco's phosphate buffered saline (DPBS) | Invitrogen | 14200075 | |
| Polystyrene disposable serological pipet | Fisher | 13-678-11D | |
| Agarose | Sigma | A9539-50G | |
| Microliter glass capillary tube (701 µm) | Fisher | 21-170J | The diameter may be varied according to the desired size for the micro-column shell. |
| Microcap bulb dispenser | Fisher | 21-170J | Bulb comes with the microcap tubes. |
| Hot plate | Fisher | SP88857200 | |
| Magnetic bar | Fisher | 1451352 | |
| Micropipette | Sigma | Z683884-1EA | |
| 25 mm gauge needle | Fisher | 14-826-49 | |
| Microscalpel | Roboz Surgical | RS-6270 | |
| Scissors | Fine Science Tools | 14081-09 | |
| Forceps | World Precision Instruments | 501985 | |
| Hot bead sterilizer | Sigma | Z378550-1EA | |
| Stereoscope | Nikon | SMZ800N | |
| Micro-spatula | Fisher | S50821 | |
| Rat tail type I collagen | Corning | 354236 | Maintain at 4 ºC and remove only when needed. Use ice to preserve its temperature when in use. |
| Microcentrifuge tube | Fisher | 02-681-256 | |
| Sodium hydroxide (NaOH) | Fisher | SS2661 | |
| Hydrochloric acid (HCl) | Fisher | SA48-1 | |
| Litmus paper | Fisher | 09-876-18 | |
| Hank's balanced salt solution (HBSS) | Invitrogen | 14170112 | Store at 4 ºC. |
| 0.25% Trypsin-EDTA | Invitrogen | 25200056 | Store at -20 ºC and warm at 37 ºC before use. |
| Bovine pancreatic deoxyribonuclease (Dnase) I | Sigma | 10104159001 | Store at -20 ºC and warm at 37 ºC before use. |
| Dulbecco's Modified Eagle Medium (DMEM) with Ham's F-12 Nutrient Mixture | Gibco | 11330-032 | Store at 4 ºC. |
| Fetal bovine serum (FBS) | Atlanta Biologicals | S11195 | Store at -20ºC. |
| Postnatal day 0 or day 1 Sprague Dawley rat pups | Charles River | Strain 001 | |
| Neurobasal embryonic neuron basal medium | Invitrogen | 21103049 | Store at 4ºC and warm at 37 ºC before use. |
| B-27 serum free supplement | Invitrogen | 12587010 | Store at -20 ºC and warm at 37 ºC before use. |
| L-glutamine | Invitrogen | 35050061 | Store at -20 ºC and warm at 37 ºC before use. |
| G5 astrocytic supplement | Invitrogen | 17503012 | |
| Sprague Dawley embryonic day 18 rats | Charles River | Strain 001 | |
| Pasteur pipette | Fisher | 22-042816 | |
| 15 mL centrifuge tube | EMESCO | 1194-352099 | |
| Vortex | Fisher | 02-215-414 | |
| Centrifuge | Fisher | 05-413-115 | |
| Hemocytometer | Fisher | 02-671-6 | |
| Incubator | Fisher | 13 998 076 | |
| Formaldehyde 40% | Fisher | F77P-4 | Formaldehyde is a toxic compound known to be carcinogenic, and must be disposed of in a separate container. |
| Glass cover slip | Fisher | 12-548-5M | |
| Nail polish | Electron Microscopy Sciences (EMS) | 72180 | |
| Fluoromont mounting medium | Southern Biotech | 0100-01 | |
| Poly-L-lysine | Sigma | P4707 | |
| Phosphate buffered saline | Fisher | BP3994 | |
| Triton X-100 | Sigma | T8787 | |
| Normal horse serum | Gibco | 16050-122 | |
| Rabbit anti-glial acidic fibrillary protein (GFAP) primary antibody | Millipore | AB5804 | Store at -20ºC. |
| Mouse anti-beta-tubulin III primary antibody | Sigma | T8578 | Store at -20ºC. |
| Rabbit anti-collagen I primary antibody | Abcam | ab34710 | Store at -20ºC. |
| Rabbit anti-vimentin | Millipore | AB3400 | Store at -20ºC. |
| Mouse anti-nestin | Millipore | AB5326 | Store at -20ºC. |
| Donkey anti-mouse 568 secondary antibody | Invitrogen | A10037 | Store at 4ºC. |
| Donkey anti-rabbit 568 secondary antibody | Invitrogen | A10042 | Store at 4ºC. |
| Donkey anti-rabbit 488 secondary antibody | Invitrogen | A21206 | Store at 4ºC. |
| Hoechst 33342, Trihydrochloride | Invitrogen | H3570 | Store at 4ºC. Hoechst is a known mutagen that should be treated as a carcinogen. Therefore, it must be disposed of in a separate container. |
| Calcein AM | Sigma | C1359 | 4 mM in anhydrous DMSO |
| Ethidium homodimer-1 | Life Technologies | E1169 | 2 mM in DMSO/H2O 1:4 (v/v) |
| Dimethyl sulfoxane (DMSO) | Sigma | 276855 | |
| A1RSI Laser Scanning Confocal Microscope | Nikon | ————– | Used for taking the confocal reconstructions of immunolabeled constructs. |
| Eclipse Ti-S Microscope | Nikon | ————– | Used for taking the phase-contrast images. With digital image acquisition using a QiClick camera interfaced with Nikon Elements Basic Research software (4.10.01). |
References
- Horner, P. J., Gage, F. H. Regenerating the damaged central nervous system. Nature. 407 (6807), 963-970 (2000).
- Yiu, G., He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 7 (8), 617-627 (2006).
- Montani, L., Petrinovic, M. M. Targeting Axonal Regeneration: The Growth Cone Takes the Lead. J. Neurosci. 34 (13), 4443-4444 (2014).
- Struzyna, L. A., Harris, J. P., Katiyar, K. S., Chen, H. I., Cullen, D. K. Restoring nervous system structure and function using tissue engineered living scaffolds. Neural Regen. Res. 10 (5), 679-685 (2015).
- Li, X., Katsanevakis, E., Liu, X., Zhang, N., Wen, X. Engineering neural stem cell fates with hydrogel design for central nervous system regeneration. Prog. Polym. Sci. 37 (8), 1105-1129 (2012).
- Lie, D. C., Song, H., Colamarino, S. A., Ming, G., Gage, F. H. Neurogenesis in the Adult Brain: New Strategies for Central Nervous System Diseases. Annu. Rev. Pharmacol. Toxicol. 44, 399-421 (2004).
- Gao, Y., Yang, Z., Li, X. Regeneration strategies after the adult mammalian central nervous system injury-biomaterials. Regen. Biomater. 3 (2), 115-122 (2016).
- Huebner, E. A., Strittmatter, S. M. Axon Regeneration in the Peripheral and Central Nervous Systems. Results Probl. Cell Differ. 48, 339-351 (2009).
- Benowitz, L. I., Yin, Y. Combinatorial Treatments for Promoting Axon Regeneration in the CNS: Strategies for Overcoming Inhibitory Signals and Activating Neurons’ Intrinsic Growth State. Dev. Neurobiol. 67 (9), 1148-1165 (2007).
- Kyungsuk, K., Liu, K., et al. Promoting Axon Regeneration in the Adult CNS by Modulation of the PTEN / mTOR Pathway. Science. 322 (5903), 963-966 (2008).
- Khakh, B. S., Sofroniew, M. V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18 (7), 942-952 (2015).
- Cregg, J. M., DePaul, M. A., Filous, A. R., Lang, B. T., Tran, A., Silver, J. Functional regeneration beyond the glial scar. Exp. Neurol. 253, 197-207 (2014).
- Buffo, A., Rolando, C., Ceruti, S. Astrocytes in the damaged brain: Molecular and cellular insights into their reactive response and healing potential. Biochem. Pharmacol. 79 (2), 77-89 (2010).
- Silver, J., Miller, J. H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5 (2), 146-156 (2004).
- Toy, D., Namgung, U. Role of Glial Cells in Axonal Regeneration. Exp. Neurobiol. 22 (2), 68-76 (2013).
- Sofroniew, M. V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32 (12), 638-647 (2009).
- East, E., de Oliveira, D. B., Golding, J. P., Phillips, J. B. Alignment of astrocytes increases neuronal growth in three-dimensional collagen gels and is maintained following plastic compression to form a spinal cord repair conduit. Tissue Eng. Part A. 16 (10), 3173-3184 (2010).
- David, S., Aguayo, A. J. Axonal Elongation into Peripheral Nervous System "Bridges" after Central Nervous System Injury in Adult Rats. Science. 214 (4523), 931-933 (1981).
- Benfey, M., Aguayo, A. J. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature. 296 (11), 150-152 (1982).
- Fry, E. J., Chagnon, M. J., López-Vales, R., Tremblay, M. L., David, S. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia. 58 (4), 423-433 (2010).
- Lin, B., Xu, Y., Zhang, B., He, Y., Yan, Y., He, M. -. C. MEK inhibition reduces glial scar formation and promotes the recovery of sensorimotor function in rats following spinal cord injury. Exp. Ther. Med. 7 (1), 66-72 (2014).
- Bradbury, E. J., Carter, L. M. Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury. Brain Res. Bull. 84 (4-5), 306-316 (2011).
- Vadivelu, S., Stewart, T. J., et al. NG2+ Progenitors Derived From Embryonic Stem Cells Penetrate Glial Scar and Promote Axonal Outgrowth Into White Matter After Spinal Cord Injury. Stem Cells Transl. Med. 4, 401-411 (2015).
- Nishimura, Y., Natsume, A., et al. Interferon-beta delivery via human neural stem cell abates glial scar formation in spinal cord injury. Cell Transplant. 22 (12), 2187-2201 (2013).
- Guo, Z., Zhang, L., Wu, Z., Chen, Y., Wang, F., Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 14 (2), 188-202 (2014).
- Tam, R. Y., Fuehrmann, T., Mitrousis, N., Shoichet, M. S. Regenerative therapies for central nervous system diseases: a biomaterials approach. Neuropsychopharmacology. 39 (1), 169-188 (2014).
- Cullen, D. K., Tang-Schomer, M. D., et al. Microtissue engineered constructs with living axons for targeted nervous system reconstruction. Tissue Eng. Part A. 18 (21-22), 2280-2289 (2012).
- Cullen, D. K., Wolf, J. A., Vernekar, V., Vukasinovic, J., LaPlaca, M. C. Neural tissue engineering and biohybridized microsystems for neurobiological investigation in vitro (Part 1). Crit. Rev. Biomed. Eng. 39 (3), 201-240 (2011).
- Irons, H. R., Cullen, D. K., Shapiro, N. P., Lambert, N. A., Lee, R. H., Laplaca, M. C. Three-dimensional neural constructs: a novel platform for neurophysiological investigation. J. Neural Eng. 5 (3), 333-341 (2008).
- Morrison, B. I., Cullen, D. K., LaPlaca, M. In Vitro Models for Biomechanical Studies of Neural Tissues. Stud. Mechanobiol. Tissue Eng. Biomater. 3, 247-285 (2011).
- Vukasinovic, J., Cullen, D. K., Laplaca, M. C., Glezer, A. A microperfused incubator for tissue mimetic 3D cultures. Biomed. Microdevices. 11 (6), 1155-1165 (2009).
- Cullen, D. K., Lessing, M. C., Laplaca, M. C. Collagen-dependent neurite outgrowth and response to dynamic deformation in three-dimensional neuronal cultures. Ann. Biomed. Eng. 35 (5), 835-846 (2007).
- LaPlaca, M. C., Vernekar, V. N., Shoemaker, J. T., Cullen, D. K. Three-dimensional neuronal cultures. Methods Bioeng. 3D Tissue Eng. , (2010).
- Chwalek, K., Tang-Schomer, M. D., Omenetto, F. G., Kaplan, D. L. In vitro bioengineered model of cortical brain tissue. Nat. Protoc. 10 (9), 1362-1373 (2015).
- Struzyna, L. A., Katiyar, K., Cullen, D. K. Living scaffolds for neuroregeneration. Curr. Opin. Solid State Mater. Sci. 18 (6), 308-318 (2014).
- Stiles, J., Jernigan, T. L. The basics of brain development. Neuropsychol. Rev. 20 (4), 327-348 (2010).
- Kaneko, N., Marín, O., et al. New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron. 67 (2), 213-223 (2010).
- Hidalgo, A., Booth, G. E. Glia dictate pioneer axon trajectories in the Drosophila embryonic CNS. Development. 127 (2), 393-402 (2000).
- Chotard, C., Salecker, I. Neurons and glia: Team players in axon guidance. Trends Neurosci. 27 (11), 655-661 (2004).
- Wang, C., Liu, F., et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 21 (11), 1534-1550 (2011).
- Peretto, P., Giachino, C., Aimar, P., Fasolo, A., Bonfanti, L. Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain. J. Comp. Neurol. 487 (4), 407-427 (2005).
- Zukor, K. A., Kent, D. T., Odelberg, S. J. Meningeal cells and glia establish a permissive environment for axon regeneration after spinal cord injury in newts. Neural Dev. 6, (2011).
- Reier, P. J. Penetration of grafted astrocytic scars by regenerating optic nerve axons in xenopus tadpoles. Brain Res. 164 (1-2), 61-68 (1979).
- Lee-Liu, D., Edwards-Faret, G., Tapia, V. S., Larraín, J. Spinal cord regeneration: Lessons for mammals from non-mammalian vertebrates. Genesis. 51 (8), 529-544 (2013).
- Goldshmit, Y., Sztal, T. E., Jusuf, P. R., Hall, T. E., Nguyen-Chi, M., Currie, P. D. Fgf-Dependent Glial Cell Bridges Facilitate Spinal Cord Regeneration in Zebrafish. J. Neurosci. 32 (22), 7477-7492 (2012).
- Struzyna, L. A., Wolf, J. A., et al. Rebuilding Brain Circuitry with Living Micro-Tissue Engineered Neural Networks. Tissue Eng. Part A. 21 (21-22), 2744-2756 (2015).
- Harris, J. P., Struzyna, L. A., Murphy, P. L., Adewole, D. O., Kuo, E., Cullen, D. K. Advanced biomaterial strategies to transplant preformed micro-tissue engineered neural networks into the brain. J. Neural Eng. 13 (1), 16019-16037 (2016).
- Huang, J. H., Cullen, D. K., et al. Long-Term Survival and Integration of Transplanted Engineered Nervous Tissue Constructs Promotes Peripheral Nerve Regeneration. Tissue Eng. Part A. 15 (7), 1677-1685 (2009).
- Winter, C. C., Katiyar, K. S., et al. Transplantable living scaffolds comprised of micro-tissue engineered aligned astrocyte networks to facilitate central nervous system regeneration. Acta Biomater. 38, 44-58 (2016).
- Alexander, J. K., Fuss, B., Colello, R. J. Electric field-induced astrocyte alignment directs neurite outgrowth. Neuron Glia Biol. 2 (2), 93-103 (2006).
- Hsiao, T. W., Tresco, P. A., Hlady, V. Astrocytes alignment and reactivity on collagen hydrogels patterned with ECM proteins. Biomaterials. 39, 124-130 (2015).
- Alekseeva, T., Katechia, K., Robertson, M., Riehle, M. O., Barnett, S. C. Long-term neurite orientation on astrocyte monolayers aligned by microtopography. Biomaterials. 28 (36), 5498-5508 (2007).
- Pacifici, M., Peruzzi, F. Isolation and culture of rat embryonic neural cells: a quick protocol. J. Vis. Exp. (63), (2012).
- Conway, A., Schaffer, D. V. Biomaterial microenvironments to support the generation of new neurons in the adult brain. Stem Cells. 32 (510), 1220-1229 (2014).
- Barry, D., McDermott, H. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 50 (3), 187-197 (2005).
- Pertusa, M., Garcia-Matas, S., Rodriguez-Farre, E., Sanfeliu, C., Cristofol, R. Astrocytes aged in vitro show a decreased neuroprotective capacity. J. Neurochem. 101 (3), 794-805 (2007).
- Discher, D. E., Janmey, P., Wang, Y. -. L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science. 310 (5751), 1139-1143 (2005).
- Balgude, A. P., Yu, X., Szymanski, A., Bellamkonda, R. V. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 22 (10), 1077-1084 (2001).
- Smeal, R. M., Tresco, P. A. The influence of substrate curvature on neurite outgrowth is cell type dependent. Exp. Neurol. 213 (2), 281-292 (2008).
- Smeal, R. M., Rabbitt, R., Biran, R., Tresco, P. A. Substrate curvature influences the direction of nerve outgrowth. Ann. Biomed. Eng. 33 (3), 376-382 (2005).
- Jain, A., Kim, Y. -. T., McKeon, R. J., Bellamkonda, R. V. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 27 (3), 497-504 (2006).
- Katiyar, K. S., Winter, C. C., Struzyna, L. A., Harris, J. P., Cullen, D. K. Mechanical elongation of astrocyte processes to create living scaffolds for nervous system regeneration. J. Tissue Eng. Regen. Med. , (2016).
- McCarthy, K. D., De Vellis, J. Preparation of Separate Astroglial and Oligodendroglial Cell Cultures from Rat Cerebral Tissue. J. Cell Biol. 85, 890-902 (1980).
- Cullen, D. K., Stabenfeldt, S. E., Simon, C. M., Tate, C. C., LaPlaca, M. C. In Vitro Neural Injury Model for Optimization of Tissue-Engineered Constructs. J. Neurosci. Res. 85, 3642-3651 (2007).
- Kim, S. U., Stern, J., Kim, M. W., Pleasure, D. E. Culture of purified rat astrocytes in serum-free medium supplemented with mitogen. Brain Res. 274 (1), 79-86 (1983).
- Morrison, R. S., de Vellis, J. Growth of purified astrocytes in a chemically defined medium. Proc. Natl. Acad. Sci. U. S. A. 78 (11), 7205-7209 (1981).
- Stopak, D., Harris, A. K. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev. Biol. 90 (2), 383-398 (1982).
- Hsiao, T. W., Swarup, V. M., Kuberan, B., Tresco, P. A., Hlady, V. Astrocytes specifically remove surface-adsorbed fibrinogen and locally express chondroitin sulfate proteoglycans. Acta Biomater. 9 (7), 7200-7208 (2013).
- Phillips, J. B., Bunting, S. C. J., Hall, S. M., Brown, R. A. Neural tissue engineering: a self-organizing collagen guidance conduit. Tissue Eng. 11 (9), 1611-1617 (2005).
- Cullen, D. K., Simon, C. M., LaPlaca, M. C. Strain rate-dependent induction of reactive astrogliosis and cell death in three-dimensional neuronal-astrocytic co-cultures. Brain Res. 1158, 103-115 (2007).
- Filous, A. R., Miller, J. H., Coulson-Thomas, Y. M., Horn, K. P., Alilain, W. J., Silver, J. Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev. Neurobiol. 70 (12), 826-841 (2010).
- Johansson, S., Strömberg, I. Guidance of dopaminergic neuritic growth by immature astrocytes in organotypic cultures of rat fetal ventral mesencephalon. J. Comp. Neurol. 443 (3), 237-249 (2002).
- Jiang, Z., Han, Y., Cao, X. Induced pluripotent stem cell (iPSCs) and their application in immunotherapy. Cell. Mol. Immunol. 11 (1), 17-24 (2014).
- Wang, L., Cao, J., et al. Immunogenicity and functional evaluation of iPSC-derived organs for transplantation. Cell Discov. 1, (2015).
- Wolmer-Solberg, N., Cederarv, M., Falci, S., Odeberg, J. Human neural stem cells and astrocytes, but not neurons, suppress an allogeneic lymphocyte response. Stem Cell Res. 2 (1), 56-67 (2009).

