An Immunohistopathologic Study to Profile the Folate Receptor Beta Macrophage and Vascular Immune Microenvironment in Giant Cell Arteritis
Summary
The protocol illustrates the use of histopathologic examination and immunohistochemistry to profile the folate receptor beta macrophage and its relationship with the total immune cell infiltrate in temporal artery biopsies in giant cell arteritis.
Abstract
Giant cell arteritis (GCA) is a chronic immune-mediated disease of medium-to-large sized arteries that affects older adults. GCA manifests with arthritis and occlusive symptoms of headaches, stroke or vision loss. Macrophages and T-helper lymphocytes infiltrate the vascular wall and produce a pro-inflammatory response that lead to vessel damage and ischemia. To date, there is no GCA biomarker that can monitor disease activity and guide therapeutic response.
Folate receptor beta (FRB) is a glycosylphosphatidylinositol protein that is anchored on cell membranes and normally expressed in the myelomonocytic lineage and in the majority of myeloid leukemia cells as well as in tumor and rheumatoid synovial macrophages, where its expression correlates with disease severity. The ability of FRB to bind folate compounds, folic acid-conjugates and antifolate drugs has made it a druggable target in cancer and inflammatory disease research. This report describes the histopathologic and immunohistochemical methods used to assess expression and distribution of FRB in relation to GCAimmunopathology.
Formalin-fixed and paraffin-embedded temporal artery biopsies from GCA and normal controls were stained with Hematoxylin and Eosin to review tissue histology and identify pathognomonic features.Immunohistochemistry was used to detect FRB, CD68 and CD3 expression. A microscopic analysis was performed to quantify the number of positively stained cells on 10 selected high-power-field sections and their respective locations in the arterial wall.
Lymphohistiocytic (LH) inflammation accompanied by intimal hyperplasia and disrupted elastic lamina was seen in GCA with none found in controls. The LH infiltrate was composed of approximately 60% lymphocytes and 40% macrophages. FRB expression was restricted to macrophages, comprising 31% of the total CD68+ macrophage population and localized to the media and adventitia. No FRB was seen in controls.
This protocol demonstrated a distinct numerical and spatial pattern of the FRB macrophage relative to the vascular immune microenvironment in GCA.
Introduction
Giant cell arteritis (GCA) is an inflammatory disease of medium-to-large arteries, targeting the aorta and its branches and affecting older adults. It presents with mild to severe ischemic complications such as headaches, jaw pain, vision loss, stroke and tissue gangrene. The diagnosis is confirmed by high inflammatory markers like erythrocyte sedimentation rate (ESR) and a distinct histopathologic pattern on the temporal artery biopsy (TAB)1. GCA is the most common adult vasculitis, and the accessibility of the temporal artery obtained for diagnosis presents an advantage over other vasculopathies, thus enabling one to study its pathogenesis more readily. The typical findings on TAB include an infiltrate of macrophages/histiocytes and T lymphocytes found across all vascular layers of the tunica intima, media, and adventitia with concurrent destruction of the elastic lamina that normally separates these compartments2. The current evidence demonstrates that GCA involves an unknown antigen that activates dendritic cells in the vascular adventitia, followed by the recruitment of helper T (Th) cells, specifically Th1 and Th17 subtypes which secrete interleukin-17 and interferon-gamma (IFG) respectively. IFG then recruits and activates macrophages to produce cytokines and proteases. These include pro-inflammatory cytokines; including IL-12, which reciprocally activates Th1 cells thus providing a positive feedback loop; tumor necrosis factor and interleukin-6, which cause arthritis and fevers and metalloproteases that damage the elastic lamina. Glucocorticoids (GC), which constitute the standard chronic therapy, partially control the cytokine pathways by attenuating the Th17/IL-17 but not the Th1/IFG arm of the immune response3,4. Unfortunately, discontinuation of GC results in disease relapse. As an alternative modality, methotrexate has been repurposed for GCA treatment, but the desired clinical endpoints were not consistently achieved although more sensitive biomarkers have not been utilized for therapeutic monitoring5,6. In 2017, the interleukin 6 blocker, tocilizumab, was approved for the treatment of GCA as it effectively demonstrated disease control and steroid sparing effects7,8.
To date, there are no good biomarkers for GCA. As a result, it is difficult to monitor disease activity and titrate doses of available drugs to avoid adverse effects and reduce the burden of cost to society. Thus, it is imperative to look for a candidate biomarker that correlates with disease activity, provide novel insights on pathogenesis and aid in therapeutic decisions.
The dysregulation of macrophage activation is a critical factor in GCA pathogenesis. Thus, an effective means of treating GCA may be the selective targeting of activated macrophages. The folate receptor beta (FRB) is a glycosyl-phosphatidylinositol glycoprotein that is anchored on the cell membrane expressed in normal myelomonocytic cells, myeloid leukemia cells and activated macrophages. Its ligand, folic acid, is an essential vitamin in its reduced form, which enables cellular DNA synthesis, methylation and repair9. FRB expression is induced in autoimmune disease and during carcinogenesis. Its expression is restricted to the surface of monocytes or pro-inflammatory M1 macrophages in rheumatoid arthritis, or to anti-inflammatory M2 macrophages in solid organ and myeloid malignancies10,11,12,13. In addition to its potential role as a biomarker of activated macrophages, the FRB can enable selective drug transport thru a multi-step process that starts with the receptor binding to folic acid, antifolates or folate-conjugated drugs at the cell surface, followed by internalization, release and eventual delivery of desired drug to the internal cell machinery12,14,15,16. In contrast to FRB expressed in cancer cells and activated macrophages, FRB expressed in normal hematopoietic cells is unable to bind folates thereby ensuring that FRB/folate-mediated drug delivery is limited to FRB-positive inflammatory or cancer cells.
In our previous study, we demonstrated for the first time that FRB is expressed in GCA macrophages and its expression correlated with CD68+ and endothelin receptor beta macrophages, which contribute to GCA pathogenesis17. In this report, we describe the histopathological and immunohistochemical methods used to assess the FRB macrophage, and analyzed the total lymphocyte and macrophage counts to gain insight into the FRB's position in the GCA vascular landscape.
Protocol
All methods described were approved by the Penn State Institutional Review Board.
1. Histopathological Preparation, and Processing After Obtaining Temporal Artery Biopsy
NOTE: The temporal artery biopsy (TAB) is performed under local anesthesia and sterile conditions by a certified surgeon. After surgically obtaining a 3 cm arterial section on the more affected side, the specimen is fixed in formalin immediately for 24 h, divided into 3 to 4 mm sections, and embedded in paraffin, paying careful attention to the proper alignment of tissue in the block which is then stored at room temperature. Specimen selection is performed after verifying the medical records. The diagnosis is based on the American College of Rheumatology 1990 criteria which requires that subjects fulfill 3 of 5 domains: age >50 years, new headache, temporal artery tenderness, an erythrocyte sedimentation rate >50 mm/hour by Westergren method and an abnormal TAB. For safety, protective gloves must be worn at all times when handling the specimens, chemicals and antibodies.
- From the embedded blocks, cut 4-5 µm thick sections using a microtome. and stain with Hematoxylin and Eosin (H&E). Use a control section of selected normal tissue for quality assurance.
- Float cut sections on a warm water bath heated to 40 °C to remove wrinkles and pick up with a coated glass microscopic slide. Place the glass slides on a warm plate in a 60 °C oven for 60 minutes to facilitate drying and adherence of section to the slide.
- Place the glass slides on a warm plate in a 60 °C oven for 60 minutes to facilitate drying and adherence of section to the slide.
- Mount 3 sections on microscope slides.
- Place slides in a vertical rack and dry overnight at 37 °C for 24 h.
- Place slides in a container and store at 4 °C.
2. Immunohistochemical Preparation, Dewaxing, Antigen Retrieval and Staining15,18,19,20
- Load slides into a slide rack. Run the rack through dishes as follows: Twice in 100% xylene for 5 minutes with 10 seconds of gentle agitation every 30 seconds, once in 100% ethanol with 10 seconds agitation, once in 90% ethanol with 10 seconds agitation, once in 70% ethanol with 10 seconds agitation, and twice in double distilled H2O with 10 seconds agitation.
Note: Handling of xylene and acetone should be done in ventilated hoods to avoid inhalation and respiratory toxicity. - Retrieve the antigen by transferring the rack into a glass container with 200 mL of 10 mmol/L, pH 6.0 citrate buffer prewarmed to 95 °C. Set the timer for 30 minutes in the water bath, then cool in room temperature for 20 minutes then rinse with running water for 5 minutes.
- Remove endogenous peroxidase activity by incubating in 200 mL of 3% hydrogen peroxide for 10 minutes. Wash slides 3 times in 200 µL of Tris-buffered saline solution (TBSS).
- Remove the slides from the rack and dab each one gently to wipe excess buffer. For staining, add 200 µL of the following primary antibodies, add cover slips on slides and incubate in a humid chamber for 1 hour at room temperature:
Anti-FRB antibody diluted 1:800
Monoclonal mouse anti-human CD68, 1:200 dilution
Polyclonal rabbit anti-CD3 1:100 dilution
Note: Formalin-fixed paraffin-embedded sections of placenta were also processed as outlined in Steps 2.1 to 2.9 and incubated with anti-FRB18 and used as positive control. Staining results were obtained by finding the optimal antibody concentration and was performed by titrating the antibody in double dilutions (e.g. 1:50, 1:100, 1:200, 1:400, 1:800, 1:1600). - Remove cover slip after incubation and discard. Rinse slides 3 times for 2 minutes each with TBSS, drain and wipe excess buffer.
- Add 200 µL of secondary antibody solution containing biotinylated Anti-Rabbit/Mouse secondary antibody to react with unconjugated primary antibody bound to tissue antigen. Place coverslips on slides and incubate in a humid chamber for 45 minutes at room temperature.
- Remove cover slip after incubation and discard. Rinse slides 3 times for 2 minutes each with TBS, drain and wipe excess buffer.
- Add 200 µL of diaminobenzidine (DAB)+substrate buffer (Imidazole HCl) -chromogen system and incubate for 10 minutes to visualize staining pattern. Wash with TBSS then in running tap water for 10 minutes.
- Counterstain with hematoxylin for 3 minutes.
- Mount the slides by applying 70 µL of mounting medium to the surface of the slide. Slowly tip the coverslip onto the mounting medium and avoid creating bubbles as you lower it into place. Wait 24 hours to dry.
3. Histopathologic and Immunohistochemical (IHC) Analysis
Examine the H&E and IHC stained sections from GCA positive and normal subjects using a light microscope.
- For the H&E stained sections:
- Assess the vascular architecture and identify endothelial cells in the tunica intima, smooth muscle cells in the tunica media, and fibroblasts and vasa vasorum in the tunica adventitia21,22.
- Identify GCA features, which include lymphocyte and macrophage/histiocyte infiltration, cellular hyperplasia, and internal elastic lamina degradation.
- Analyze the lymphohistiocytic infiltrate by identifying and quantifying the number of macrophages relative to the lymphocytes. Assess the extent of internal elastic lamina disruption and hyperplastic changes in resident cells and compare with controls.
- For the IHC stained sections:
- Examine the staining pattern of FRB, taking note of its expression by a particular type or types of cells and its distribution within the vascular layers and its relationship to the total immune infiltrate, the total macrophage marker, anti-CD68, and the pan lymphocyte marker anti CD3.
- Quantify the expression of FRB, CD68 and CD3 cells by counting the positively stained cells on 10 multiple randomly selected high power fields (hpf) and record the means.
NOTE: Identification of these normal and pathologic cells and structures were performed by a certified cardiovascular pathologist (J.M.) and rheumatologist (S.A.A.) and the results were recorded for all cases. - Capture representative images using a digital camera.
NOTE: After these steps, remaining aliquots may be stored at -80 °C.
Representative Results
Histopathologic findings
The H&E stains in normal specimens demonstrated normal arterial anatomy with endothelial cells in the tunica intima, smooth muscle cells in the tunica media, and a heterogenous collagen matrix which include fibroblasts and feeder vessels called vasa vasorum in the tunica adventitia. There is no inflammatory infiltrate identified. GCA positive temporal arteries demonstrated moderate to severe inflammation with aggregates of macrophages/histiocytes, lymphocytes, occasional plasma cells, and multinucleated giant cells. Luminal narrowing was noted and accompanied by intimal thickening and 90% disruption of the internal elastic lamina (Figure 1).
Immunohistochemical findings
CD3+ lymphocytes and CD68+ macrophages were present in all GCA+ specimens and demonstrated similar staining patterns in all biopsies. The lymphocytes predominated over macrophages with a CD3/CD68 ratio of 1.6. Staining for FRB was restricted to macrophages and multinucleated giant cells which preferentially localized to the adventitia and media (Table 1 and Figure 2). Control specimens showed minimal leukocytes (<5 cells/high-power field) and no FRB expression.
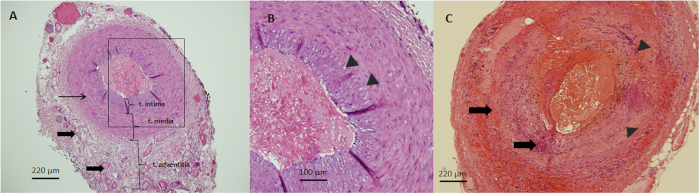
Figure 1: H&E images of temporal arteries. (A) Representative H&E image of a normal temporal artery with distinct layers of the tunica (t) adventitia, media, and intima from the outermost to the innermost/Luminal side respectively. Note the network of collagen and vasa vasorum in the adventitia (thick arrows), the smooth muscle cells in the media (thin arrows), and the endothelial cells in the intima (10x magnification). (B) An enlarged micrograph of the intima-media interface is highlighted by the rectangular inset in A and is separated by the internal elastic lamina (arrowheads) (20x magnification). (C) Representative H&E image of a TA with giant cell arteritis marked by a transmural lymphohistiocytic infiltrate (thick arrows), intimal hyperplasia and destruction of the internal elastic lamina (arrowheads) (10x magnification). Please click here to view a larger version of this figure.
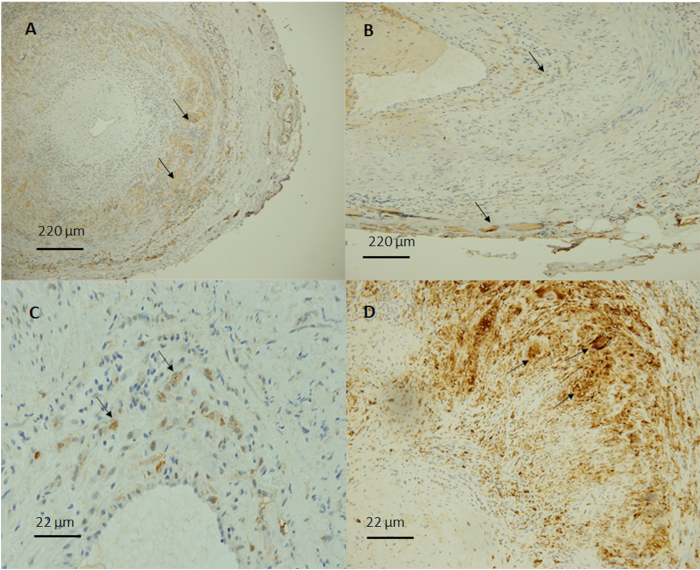
Figure 2: Immunohistochemical staining of temporal arteries with giant cell arteritis. (A) Representative images demonstrate an extensive inflammatory infiltrate with CD68 positive macrophages and giant cells (arrows) found mostly in the media and adventitia (10x magnification). (B) Folate receptor beta (FRB) was selectively expressed in activated macrophages (arrows) (10x magnification). Image taken at higher power demonstrated FRB positive macrophages in (C) and CD68 positive macrophages in (D) (100x magnification). Please click here to view a larger version of this figure.
| GCA subjects (n=5) | Mean (±SD) |
| CD3 (% of immune infiltrate) | 61.7 ± 4.1 |
| CD68 (% of immune infiltrate) | 38.3 ± 4.1 |
| CD68 histiocytes/macrophages (cells/hpf) | 34.8 ± 10.4 |
| FRB+ macrophages (cells/.hpf) | 10.8 ± 4.4 |
| %FRB/%CD68 | 31 ± 12.7 |
Table 1: Summary of FRB, CD68, and CD3 expression.
Discussion
GCA is the most common vasculitis in adults, and its pathologic hallmark exemplifies a potent combination of T helper lymphocytes and activated macrophages that can also be found in other granulomatous diseases or vasculopathies like sarcoidosis and granulomatous polyangiitis2,3. In GCA, the temporal artery provides a good representation of a medium-to-large sized artery and the TA biopsy gives a sizeable sample that can be stored in paraffin blocks for years, thus creating opportunities to study the roles of emerging diagnostic and therapeutic targets like FRB in the vasculitic microenvironment. We took the advantage presented by accessible tissue and standard immunopathologic methods to ask whether the FRB can be reliably analyzed through IHC. Our findings demonstrate that histopathology and IHC can serve as valuable tools not only in confirming the diagnosis and extent of vascular damage but also in validating the role of different macrophage subtypes in GCA.
The histopathology and IHC methods are both labor intensive and require the skills of the histochemical technician, pathologist and rheumatologist, but the techniques have been well established and can serve as a launching pad for using other methods such as immunofluorescence and flow cytometry. The disadvantage of such methods, however, is the need for fresh frozen tissue which requires prospective collection. The IHC, together with histopathologic examination, is invaluable in presenting a panoramic perspective of the vascular immune microenvironment that can be repeatedly accessed and analyzed especially with the emergence of novel proteins like the FRB. The Hematoxylin and Eosin (H&E) staining enables one to review tissue histology. Hematoxylin stains the nucleus and highlights the nuclear details while eosin serves as a counterstain which aids in demonstrating the differences between the nuclear and cytoplasmic features. The use of a control section of selected normal tissue is essential to evaluate for quality assurance.
The IHC detects the antigen of interest in frozen or formalin-fixed and paraffin-embedded tissues through the use of multiple antibodies which are visualized by microscopy. The protocol described was adapted from van der Heijden et al.15, who demonstrated FRB expression in synovial macrophages in rheumatoid arthritis. The essential IHC steps include tissue preparation, dewaxing/deparaffinization, antigen retrieval and staining23. It is essential to ensure that the tissue removed from the patient is immediately fixed in formalin and tissue alignment and orientation meticulously preserved when fixed in paraffin. The tissue is thinly sectioned with the use of a microtome and undergoes a dewaxing step that requires multiple washes in xylene, ethanol and water. The next step, called antigen retrieval, is needed to break down the methylene linkages formed during formalin fixation and requires a citrate-based buffer and moist heat. The slides are treated with hydrogen peroxide to block endogenous peroxidase enzymes that can interfere with antigen detection. The staining procedure is next performed by incubating the slides with diluted primary antibodies that will bind the antigen of interest followed by applying a secondary antibody that will detect the primary antigen-antibody reaction. This is followed by labeling with a chromogen DAB-substrate which forms a brown pigment that is visualized under the microscope. The final step is the careful mounting of the tissue with a permanent solution making sure that no interfering substances like bubbles are formed between the cover slip and surface. The protocol required modifications to accommodate the use of arterial tissue. Since the study was the first to evaluate FRB in GCA, the optimal antibody concentration was unknown and needed multiple dilutions at 1:50, 1:100, 1:200, 1:400, 1:800, 1:1,600, 1:3200 with the best staining quality at the lowest background noise or signal achieved at 1:800.
The membrane-associated FRB was earlier validated by Ross et al.18,24 and was found to be expressed in normal placental cells, neutrophils, leukemic blasts in chronic myelogenous leukemia, promyelocytic leukemia, myeloblast populations of myelomonocytic, and erythroleukemias and variably in M1/M2 acute myelogenous leukemia. The affinity-purified rabbit polyclonal antibody specific for FRB used in these earlier works have since been applied to studies of rheumatoid arthritis positive synovial macrophages and solid organ tumors. The FRB antibody used in this protocol is not commercially available and may hamper broad application. Conceivably, the methodology described here may also work for other FRB antibodies but will require individual optimization before its use in larger numbers. Since FRB is expressed significantly in placenta, this tissue has been repeatedly used as a positive control.
The study design was limited by a small sample size and requires validation in larger numbers of TABs ideally obtained at early and late stages of GCA. In addition, since the FRB macrophage can serve as a potential diagnostic and therapeutic target with folic conjugated agents and antifolates, the data obtained here supports the need to further examine FRB phenotype in the context of M1 and M2 macrophage polarization. With respect to other diagnostics, these results open opportunities for non-invasive imaging approaches with FRB targeted folic acid conjugated SPECT of PET imaging agents. Additionally, given that macrophage infiltration is a hallmark of disease progression and activity in atherosclerosis, scleroderma, sarcoidosis, and rheumatoid arthritis, the use of this protocol can find applications in these conditions.
This is the first protocol to explore the expression and distribution of the FRB in GCA. The IHC permitted a panoramic cross-sectional and multiple views of the TA and allows analysis of the numerical relationships between the immune infiltrate and the arterial microenvironment. The FRB comprised 30% of the total macrophages that infiltrated all layers of the vascular wall, but interestingly, the FRB was not found in the intima and localized only to the adventitia and media. This pattern of distribution may reveal novel insights on FRB macrophage tropism for the differential collagen composition found in the media and adventitial layers25. The immune infiltrate consisted of about 60% lymphocytes (as measured by the pan-lymphocyte marker anti-CD3) and 40% macrophages (marked by pan-macrophage marker anti-CD68). A recent study correlated CD3 with a higher sensitivity for GCA diagnosis while another linked CD68 expression higher disease activity. Thus, we propose that the early GCA vascular immune milieu starts with a 60:40 ratio of lymphocytes:macrophages with 30% of the macrophages being FRB positive. Whether this holds true for and consistently demonstrated in patients with early disease should be further validated in future and prospective experiments. If this cellular infiltrate distribution holds consistently, it may then serve as biomarker composite for GCA disease activity.
In conclusion, this protocol demonstrated for the first time the presence of FRB infiltration in the media and adventitia of temporal arteries in GCA. FRB macrophages represented about one third of the total macrophage population in an early and active GCA microenvironment composed of 60% lymphocytes and 40% macrophages.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was made possible by the support of Dr. Douglas Stairs, Marianne Klinger , Ann Benko, the Division of Rheumatology/Department of Medicine and the Molecular and Histopathologic Core Laboratory, Penn State University College of Medicine, Hershey PA.
Materials
| Microtome | Reichert-Jung | ||
| plain and frosted microscope slides and cover slips | Fisher Scientific | ||
| Vertical rack | Fisher Scientific | ||
| Light microscope | Olympus BX60 microscope | ||
| Xylene | |||
| Acetone in distilled water | concentrations 100%, 90%, 70% | ||
| Tris-buffered saline solution (TBS) | Dako Wash BufferS3006 | ||
| 3% hydrogen peroxide in TBS | |||
| Diaminobenzidine (DAB) | Sigma Fast Tablet set | ||
| Chemical Permount Mounting Medium | Fisher Scientific | SP-15-100 | |
| Harleco Gill's III Hematoxylin | Fiisher Scientific 23-750-019 | ||
| Harleco Eosin Y 1% Alcoholic Stock Solution | Fisher Scientific | 23-749-977 | |
| 10 mM citric acid monohydrate (pH 6.0) | |||
| Polyclonal rabbit anti-folate receptor beta | Manohar Ratnam | optimized at 1:800 | |
| Monoclonal mouse anti-human CD68 | Dako | M071801 | optimized at 1:200 |
| Polyclonal rabbit anti-CD3 | Agilent Dako | A0452 | optimized at 1:100 |
| Polymer goat anti- rabbit/mouse secondary antibody | Dako | ||
| Placental tissue | for FRB positive control |
References
- Klippel, J. H., Stone, J. H., White, P. H. . Primer on the rheumatic diseases. , (2008).
- Weyand, C. M., Goronzy, J. J. Immune mechanisms in medium and large-vessel vasculitis. Nature Reviews Rheumatol. 9 (12), 731-740 (2013).
- Weyand, C. M., Liao, Y. J., Goronzy, J. J. The immunopathology of giant cell arteritis: diagnostic and therapeutic implications. Journal of Neuro-ophthalmology. 32 (3), 259-265 (2012).
- Deng, J., Younge, B., Olshen, R., Goronzy, J., Weyand, C. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 121, 906-915 (2010).
- Mahr, A. D., et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis & Rheumatology. 56 (8), 2789-2797 (2007).
- Hoffman, G. S., et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis & Rheumatology. 46 (5), 1309-1318 (2002).
- Stone, J. H., Klearman, M., Collinson, N. Trial of Tocilizumab in Giant-Cell Arteritis. New England Journal of Medicine. 377 (15), 1494-1495 (2017).
- Milman, N. Tocilizumab increased sustained glucocorticoid-free remission from giant cell arteritis. Annals of Internal Medicine. 167 (12), JC63 (2017).
- Xia, W., et al. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 113 (2), 438-446 (2009).
- Shen, J., et al. Folate receptor-beta constitutes a marker for human proinflammatory monocytes. Journal of Leukocyte Biology. 96 (4), 563-570 (2014).
- Salazar, M. D., Ratnam, M. The folate receptor: what does it promise in tissue-targeted therapeutics. Cancer and Metastasis Reviews. 26 (1), 141-152 (2007).
- Low, P. S., Henne, W. A., Doorneweerd, D. D. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Accounts of Chemical Research. 41 (1), 120-129 (2008).
- Puig-Kroger, A., et al. Folate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Research. 69 (24), 9395-9403 (2009).
- Nakashima-Matsushita, N., et al. Selective expression of folate receptor beta and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis & Rheumatology. 42 (8), 1609-1616 (1999).
- vander Heijden, J. W., et al. Folate receptor beta as a potential delivery route for novel folate antagonists to macrophages in the synovial tissue of rheumatoid arthritis patients. Arthritis & Rheumatology. 60 (1), 12-21 (2009).
- Jansen, G., Peters, G. J. Novel insights in folate receptors and transporters: implications for disease and treatment of immune diseases and cancer. Pteridines. 26 (2), 41-53 (2015).
- Albano-Aluquin, S., Malysz, J., Aluquin, V. R., Ratnam, M., Olsen, N. An immunohistochemical analysis of folate receptor beta expression and distribution in giant cell arteritis – a pilot study. American Journal of Clinical and Experimental Immunology. 6 (6), 107-114 (2017).
- Ross, J. F., Chaudhuri, P. K., Ratnam, M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 73 (9), 2432-2443 (1994).
- Reiner, N. E. Methods in molecular biology. Macrophages and dendritic cells. Methods and protocols. Preface. Methods in Molecular Biology. 531, (2009).
- Robertson, D., Savage, K., Reis-Filho, J. S., Isacke, C. M. Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biology. 9, 13 (2008).
- Pawlina, W. . Histology: A text and atlas with correlated cell and molecular biology. , (2016).
- Kumar, V., Abbas, A., Robbins Aster, J. . Robbins and Cotran pathologic basis of disease. , (2015).
- Dabbs, D. J. . Diagnostic immunohistochemistry. , (2019).
- Ross, J. F., et al. Folate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer. 85 (2), 348-357 (1999).
- Holzapfel, G. A., Fratzl, P. Collagen in arterial walls: biomechanical aspects. Collagen. Structure and Mechanics. , 285-324 (2008).
- Sultan, H., Smith, S. V., Lee, A. G., Chevez-Barrios, P. Pathologic Markers Determining Prognosis in Patients with Treated or Healing Giant Cell Arteritis. American Journal of Ophthalmology. , (2018).

