用于制造反向电透析装置的离子交换膜
Summary
我们演示了使用离子交换膜 (CEM) 和离子交换膜 (AEM) 发电的逆向电透析设备的制造。
Abstract
反向电透析 (RED) 是一种有效的方法,使用离子交换膜 (CEM) 和离子交换膜 (AEM) 将水中的两种不同的盐浓度混合在水中。RED 堆栈由离子交换膜和离子交换膜的交替排列组成。RED 设备是满足未来能源危机普遍需求的潜在候选设备。在这里,在本文中,我们演示了使用实验室规模的 CEM 和 AEM 制造反向电透析设备用于发电的程序。离子交换膜的活性面积为49厘米2。在本文中,我们提供了一个合成膜的分步程序,然后是堆栈的组装和功率测量。还解释了测量条件和净功率输出计算。此外,我们描述了为获得可靠结果而考虑的基本参数。我们还提供一个理论参数,影响与膜和饲料溶液相关的整体细胞性能。简言之,这个实验描述了如何在同一平台上组装和测量红细胞。它还包含使用 CEM 和 AEM 膜估算 RED 堆栈净功率输出的工作原理和计算。
Introduction
从自然资源中获取能源是一种经济的方法,是环保的,从而使我们的星球绿色和清洁。到目前为止,已经提出了几个提取能量的过程,但反向电透析(RED)具有巨大的潜力来克服能源危机问题1。逆向电透析发电是全球能源脱碳的技术突破。顾名思义,RED是一个反向过程,其中备用细胞室充满了高浓度盐溶液和低浓缩盐溶液2。从隔间末端的电极收集的离子交换膜上的盐浓度差产生的化学潜力。
自2000年以来,发表了许多研究文章,从理论和实验上深入了解了红色3、4。对应力条件下的操作条件和可靠性研究进行系统研究,改进了堆栈结构,提高了整体单元格性能。一些研究小组已经将注意力转移到RED的混合应用上,如红与海水淡化工艺5,红色与太阳能6,红色与反渗透(RO)过程5,RED与微生物燃料电池7,红色与辐射冷却过程8。如前所述,在实施RED的混合应用以解决能源和清洁水问题方面有很大的空间。
采用了几种方法来提高红细胞的性能和膜的离子交换能力。使用硫酸组(-SO3H)、磷酸组(-PO3H2)和碳酸组(-COOH)定制不同类型的离子的cation交换膜是改变膜物理化学特性的有效方法之一。离子交换膜是用铵组 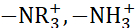 ()9量身定做的。AEM 和 CEM 的高离子电导率而不降低膜的机械强度,是选择合适的膜进行设备应用的关键参数。应力条件下的坚固膜为膜提供机械稳定性,并增强设备的耐久性。在这里,高性能独立硫化聚(乙醚醚酮)(sPEEK)作为cation交换膜与FAA-3作为离子交换膜的独特组合用于红色应用。 图1 显示了实验过程的流程图。
()9量身定做的。AEM 和 CEM 的高离子电导率而不降低膜的机械强度,是选择合适的膜进行设备应用的关键参数。应力条件下的坚固膜为膜提供机械稳定性,并增强设备的耐久性。在这里,高性能独立硫化聚(乙醚醚酮)(sPEEK)作为cation交换膜与FAA-3作为离子交换膜的独特组合用于红色应用。 图1 显示了实验过程的流程图。
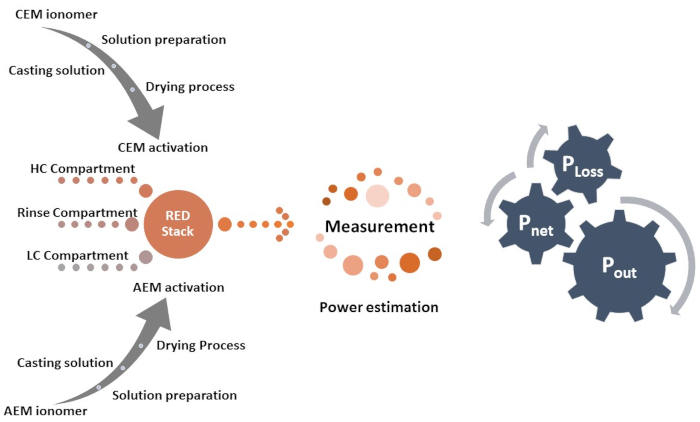
图1: 程序图。 流程图介绍了离子交换膜制备的程序,然后是反向电透析测量过程。 请单击此处查看此图的较大版本。
Protocol
Representative Results
Discussion
RED 的工作原理主要由膜的物理化学特性主导,这是 RED 系统的重要组成部分, 如图 3所示。在这里,我们描述了膜的基本特征,以提供高性能的红色系统。膜的特异性离子渗透性使其通过聚合物纳米通道传递一种离子。顾名思义,CEM 可以将离子从一侧传递到另一侧并限制离子,而 AEM 可以传递 anion 并限制离子。如图 2所示,所有膜都被塑造成红色堆栈?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
这项工作得到了韩国国家研究基金会(NRF)资助的支持,该赠款由韩国政府资助(MEST)( No.NRF-2017R1A2A2A05001329)。手稿的作者感谢大韩民国汉城的索冈大学。
Materials
| AEM based membrane | Fumion | P1810-194 | Ionomer |
| CEM based membrane | Fumion | E550 | Ionomer |
| Digital torque wrench | Torqueworld | WP2-030-09000251 | wrench |
| Labview software | Natiaonal Instrument | – | Software |
| Laptop | LG | – | PC |
| Magnetic stirrer | Lab Companion | – | MS-17BB |
| N, N-Dimethylacetamide | Sigma aldrich | 271012 | Chemical |
| N-Methyl-2- pyrrolidone | Daejung | 872-50-4 | Chemical |
| Peristaltic pump | EMS tech Inc | – | EMP 2000W |
| Potassium hexacyanoferrate(II) trihydrate | Sigma aldrich | P3289 | Chemical |
| Potassium hexacyanoferrate(III) | Sigma aldrich | 244023 | Chemical |
| Pressure Gauge | Swagelok | – | Guage |
| Reverse electrodialysis setup | fabricated in lab | – | Device |
| RO system pure water | KOTITI | – | Water |
| Rotary evaporator | Hitachi | YEFO-KTPM | Induction motor |
| Sodium Chloride | Sigma aldrich | S9888 | Chemical |
| Sodium Hydroxide | Merk | 1310-73-2 | Chemical |
| Source meter | Keithley | – | 2410 |
| Spacer | Nitex, SEFAR | 06-250/34 | Spacer |
| Sulfuric acid | Daejung | 7664-93-9 | Chemical |
| Tube | Masterflex tube | 96410-25 | Rubber tube |
References
- Dlugolecki, P., Gambier, A., Nijmeijer, K., Wessling, M. Practical potential of reverse electrodialysis as process for sustainable energy generation. Environmental Science & Technology. 43, 6888-6894 (2009).
- Kim, D., Kwon, K., Kim, D. H., Li, L. . Energy Generation Using Reverse Electrodialysis: Principles, Implementation, and Applications. , (2019).
- Mei, Y., Tang, C. Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination. 425, 156-174 (2018).
- Yip, N. Y., Brogioli, D., Hamelers, H. V. M., Nijmeijer, K. Salinity gradients for sustainable energy: primer, progress, and prospects. Environmental Science & Technology. 50, 12072-12094 (2016).
- Li, W., et al. A novel hybrid process of reverse electrodialysis and reverse osmosis for low energy seawater desalination and brine management. Applied Energy. 104, 592-602 (2013).
- Brauns, E. Salinity gradient power by reverse electrodialysis: effect of model parameters on electrical power output. Desalination. 237, 378-391 (2009).
- Cusick, R. D., Kim, Y., Logan, B. E. Energy capture from thermolytic solutions in microbial reverse-electrodialysis cells. Science. 335, 1474-1477 (2012).
- Kim, D. H., Park, B. H., Kwon, K., Li, L., Kim, D. Modeling of power generation with thermolytic reverse electrodialysis for low-grade waste heat recovery. Applied Energy. 189, 201-210 (2017).
- Hong, J. G., et al. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. Journal of Membrane Science. 486, 71-88 (2015).
- Choi, S. -. Y., et al. Controlling fuel crossover in open electrochemical cells by tuning the water nanochannel for power generation. ACS Sustainable Chemistry & Engineering. 8, 8613-8623 (2020).
- Shah, S. A., et al. Modified single-wall carbon nanotube for reducing fouling in perfluorinated membrane-based reverse electrodialysis. International Journal of Hydrogen Energy. 45, 30703-30719 (2020).
- Kwon, K., Han, J., Park, B. H., Shin, Y., Kim, D. Brine recovery using reverse electrodialysis in membrane-based desalination processes. Desalination. 362, 1-10 (2015).
- Kwon, K., Park, B. H., Kim, D. H., Kim, D. Parametric study of reverse electrodialysis using ammonium bicarbonate solution for low-grade waste heat recovery. Energy Conversion and Management. 103, 104-110 (2015).
- Hatzell, M. C., Ivanov, I., Cusick, R. D., Zhu, X., Logan, B. E. Comparison of hydrogen production and electrical power generation for energy capture in closed-loop ammonium bicarbonate reverse electrodialysis systems. Physical Chemistry Chemical Physics. 16, 1632-1638 (2014).
- Zhu, X. P., He, W. H., Logan, B. E. Reducing pumping energy by using different flow rates of high and low concentration solutions in reverse electrodialysis cells. Journal of Membrane Science. 486, 215-221 (2015).
- Vermaas, D. A., Saakes, M., Nijmeijer, K. Doubled power density from salinity gradients at reduced intermembrane distance. Environmental Science & Technology. 45, 7089-7095 (2011).
- Veerman, J., Saakes, M., Metz, S. J., Harmsen, G. J. Reverse electrodialysis: Performance of a stack with 50 cells on the mixing of sea and river water. Journal of Membrane Science. 327, 136-144 (2009).
- Veerman, J., Saakes, M., Metz, S. J., Harmsen, G. J. Electrical power from sea and river water by reverse electrodialysis: a first step from the laboratory to a real power plant. Environmental Science & Technology. 44, 9207-9212 (2010).
- Batchelor, C. K., Batchelor, G. K. . An Introduction to Fluid Dynamics. , (2000).
- Schock, G., Miquel, A. Mass transfer and pressure loss in spiral wound modules. Desalination. 64, 339-352 (1987).
- Da Costa, A. R., Fane, A. G., Wiley, D. E. Spacer characterization and pressure drop modelling in spacer-filled channels for ultrafiltration. Journal of Membrane Science. 87, 79-98 (1994).
- Vermaas, D. A., Veerman, J., Saakes, M., Nijmeijer, K. Influence of multivalent ions on renewable energy generation in reverse electrodialysis. Energy & Environmental Science. 7, 1434-1445 (2014).
- Vermaas, D. A., Saakes, M., Nijmeijer, K. Enhanced mixing in the diffusive boundary layer for energy generation in reverse electrodialysis. Journal of Membrane Science. 453, 312-319 (2014).
- Moreno, J., Grasman, S., van Engelen, R., Nijmeijer, K. Upscaling reverse electrodialysis. Environmental Science & Technology. 52, 10856-10863 (2018).
- Sarkar, S., SenGupta, A. K., Prakash, P. The donnan membrane principle: opportunities for sustainable engineered processes and materials. Environmental Science & Technology. 44, 1161-1166 (2010).
- Kim, H. -. K., et al. High power density of reverse electrodialysis with pore-filling ion exchange membranes and a high-open-area spacer. Journal of Materials Chemistry A. 3, 16302-16306 (2015).
- Długołęcki, P., Nymeijer, K., Metz, S., Wessling, M. Current status of ion exchange membranes for power generation from salinity gradients. Journal of Membrane Science. 319, 214-222 (2008).
- Geise, G. M., Curtis, A. J., Hatzell, M. C., Hickner, M. A., Logan, B. E. Salt concentration differences alter membrane resistance in reverse electrodialysis stacks. Environmental Science & Technology Letters. 1, 36-39 (2014).

