Megakaryocyte Culture in 3D Methylcellulose-Based Hydrogel to Improve Cell Maturation and Study the Impact of Stiffness and Confinement
Summary
It is now acknowledged that the three-dimensional environment of cells can play an important role in their behavior, maturation and/or differentiation. This protocol describes a three-dimensional cell culture model designed to study the impact of physical containment and mechanical constraints on megakaryocytes.
Abstract
The 3D environment leading to both confinement and mechanical constraints is increasingly recognized as an important determinant of cell behavior. 3D culture has thus been developed to better approach the in vivo situation. Megakaryocytes differentiate from hematopoietic stem and progenitor cells (HSPCs) in the bone marrow (BM). The BM is one of the softest tissues of the body, confined inside the bone. The bone being poorly extensible at the cell scale, megakaryocytes are concomitantly subjected to a weak stiffness and high confinement. This protocol presents a method for the recovery of mouse lineage negative (Lin-) HSPCs by immuno-magnetic sorting and their differentiation into mature megakaryocytes in a 3D medium composed of methylcellulose. Methylcellulose is non-reactive towards megakaryocytes and its stiffness may be adjusted to that of normal bone marrow or increased to mimic a pathological fibrotic marrow. The process to recover the megakaryocytes for further cell analyses is also detailed in the protocol. Although proplatelet extension is prevented within the 3D milieu, it is described below how to resuspend the megakaryocytes in liquid medium and to quantify their capacity to extend proplatelets. Megakaryocytes grown in 3D hydrogel have a higher capacity to form proplatelets compared to those grown in a liquid milieu. This 3D culture allows i) to differentiate progenitors towards megakaryocytes reaching a higher maturation state, ii) to recapitulate phenotypes that may be observed in vivo but go unnoticed in classical liquid cultures, and iii) to study transduction pathways induced by the mechanical cues provided by a 3D environment.
Introduction
Cells in the body experience a complex 3D microenvironment and are subjected to the interplay between chemical and mechanophysical cues including stiffness from the tissue and confinement due to neighboring cells and surrounding matrix 1,2,3. The importance of stiffness and confinement for cell behavior has only been recognized in the last decades. In 2006, the seminal work from Engler et al. 4 highlighted the importance of the mechanical environment for cell differentiation. The authors demonstrated that variation in cell substrate stiffness resulted in the orientation of stem cells towards various differentiation lineages. Since then, the impact of mechanical cues on cell fate and behavior has become increasingly recognized and studied. Despite it being one of the softest tissues of the organism, the bone marrow has a 3D structural organization that is confined inside the bone. Marrow stiffness, although technically difficult to measure precisely, is estimated to lie between 15 and 300 Pa 5, 6. Within the stroma, cells are tightly confined to one another. In addition, most of them are migrating toward the sinusoid vessels to enter the blood circulation. These conditions create additional mechanical constraints on adjacent cells, which have to adapt to these forces. Mechanical cues represent an important parameter whose consequences on megakaryocyte differentiation and proplatelet formation have just recently been explored. Although megakaryocytes can differentiate in vitro in traditional liquid culture, they do not reach the degree of maturation observed in vivo, in part due to the absence of the mechanical cues from the 3D environment 7. Growing progenitors embedded in hydrogel brings 3D mechanical cues that are lacking in liquid milieu.
Hydrogels have been widely used for several decades in the hematological field, notably to grow cells in colony forming assays to quantify hematopoietic progenitors. However, such hydrogels have seldom been used to explore the biological impact of the 3D mechanical environment on maturation and differentiation of hematopoietic cells. Over the past few years our laboratory has developed a 3D culture model using a methylcellulose-based hydrogel 8. This nonreactive physical gel is a useful tool to mimic the physical constraints of the native megakaryocyte environment. It is derived from cellulose by replacement of hydroxyl residues (-OH) by methoxide groups (-OCH3). Both the degree of methyl substitution and the methylcellulose concentration determine the hydrogel stiffness once it has jellified. During the development stage of this technique, it was demonstrated that a Young's modulus in the range of 30 to 60 Pa is the optimal gel stiffness for megakaryocyte growth 9.
The following protocol describes a method to grow mouse megakaryocytic progenitors in a 3D methylcellulose hydrogel. It has been previously shown that compared with standard liquid culture, this hydrogel culture increases the degree of megakaryocyte polyploidization, improves the maturation and intracellular organization, and increases the capacity of megakaryocytes to extend proplatelets once resuspended in a liquid medium 9. This manuscript describes in detail the protocol for the isolation of mouse bone marrow Lin− cells and their embedding in a methylcellulose hydrogel for 3D culture as well as the quantification of their capacity to produce proplatelets and the recovery of the cells for further analyses.
Protocol
All experiments should be performed in compliance with institutional guidelines for the care and use of laboratory animals. All protocols displayed in the video were carried out in strict accordance with the European law and the recommendations of the Review Board of the Etablissement Français du Sang (EFS). A first version of this protocol was originally published in 2018 in Methods in Molecular Biology 8.
NOTE: Figure 1 presents a schematic view of the whole process. This process includes 1) bone dissection, marrow retrieval, and mechanical isolation of marrow cells, 2) magnetic sorting of lineage negative (Lin-) cells, 3) seeding in liquid or methylcellulose hydrogel, and 4) resuspension of megakaryocytes grown in 3D gel for examination of proplatelet formation in liquid medium.
1. Bone collection from adult mice
NOTE: In this section, it is important to minimize microbial contamination.
- Prepare a 15 mL tube for bone collection with Dulbecco's Modified Eagle's Medium (DMEM) containing 1% of the total volume of penicillin-streptomycin-glutamin (PSG) antibiotic mix (penicillin 10000 U/mL, streptomycin 100000 µg/mL and L-glutamin 29.2 mg/mL).
NOTE: If all the mice used have the same genotype, pool all bones in the same tube containing 1 mL of DMEM – PSG 1% per number of mice. Antibiotics are important to prevent possible bacteria proliferation during the time of bone sampling. - Fill a 50 mL tube with ethanol 70% for bone disinfection and another one for rinsing instruments during the procedure. Use sterilized dissection instruments.
- Anesthetize the mice using isoflurane inhalation (4%) and rapidly proceed to cervical dislocation to euthanize the mice. Rapidly immerse the body in 70% ethanol to disinfect and avoid microbial contamination.
- Rapidly dissect out the tibias and femurs.
- Using a scalpel, cut away the epiphyses of the ankle side end for the tibia and of the hip side end for the femur.
- Immerse the bones for one second in 70% ethanol before immersing them in DMEM medium containing 1% PSG.
2. Marrow dissociation and Lin- cells isolation
NOTE: This part of the protocol is performed under a laminar flow hood. For one culture, all the wells are part of the same experiment and cannot be considered as independent biological replicates. The cells from all mice are pooled together to ensure the homogeneity of all the wells and to be able to compare them to each other while eliminating possible inter-individual variability. For independent biological replicates, the culture must be repeated.
- Place the bones in a Petri dish and rise them twice in sterile Dulbecco's phosphate-buffered saline (DPBS) to remove potential contaminants.
- Prepare DMEM – 1% PSG in a 50 mL tube.
NOTE: Provide 2 mL of DMEM – 1% PSG per mice used for the experiment. - Fill a 5 mL syringe equipped with a 21-gauge needle with DMEM – 1% PSG.
- Holding the bone with forceps, introduce only the bevel of the needle at the knee side end.
NOTE: The knee side epiphysis should remain intact from the dissection, leaving a small cavity in its center through which to insert the needle. The remaining epiphysis will maintain the bone attached to the needle during flushing. Be careful not to introduce more than the bevel in the bone as it might squash and damage the marrow. - Quickly press the syringe plunger to flush the marrow out into a 50 mL tube.
NOTE: To avoid splashes and facilitate the marrow flush and liberation place the free end of the bone on the tube wall, immersed in DMEM – 1% PSG. In practice, a volume between 500 µL and 1 mL is generally sufficient to expel the marrow from the bone. When the marrow has been totally expelled, the bone has become white. In case the marrow has not been totally expelled from the diaphysis as judged by some remaining red color, it is possible to repeat the flush with fresh medium. - Repeat steps 2.4. and 2.5. for all bones, refilling the 5 mL syringe with DMEM – 1% PSG if necessary.
- Use the same 5 mL syringe with the 21-gauge needle to transfer the total volume of medium containing flushed marrow into round bottomed 10 mL tubes.
NOTE: It is not absolutely necessary to switch to a round bottomed 10 mL tube but it makes it easier to proceed to the following dissociation steps. Do not hesitate to change syringe and/or needle if risk of contamination is suspected. - Proceed to cell dissociation by aspirating and expelling the medium and marrow cells successively two times through a 21-gauge needle, three times through a 23-gauge and once through a 25-gauge needle.
NOTE: Avoid air bubbles as it may be detrimental for the cells. - Transfer the suspension into 15 mL tubes.
- Measure cell number and check the viability using an automated cell counter or a cell chamber for manual counting in the presence of trypan blue to exclude dead cells.
- Centrifuge the 15 mL tubes for 7 min at 300 x g. Using a 1 mL transfer pipette, carefully pipette out and discard the supernatant.
- Isolate stem and progenitor cells by negative immunomagnetic sorting using a mouse hematopoietic cell isolation kit.
NOTE: The aim of this cell sorting is to retrieve the cells that are negative for all the selection antibodies (CD5, CD11b, CD19, CD45R/B220, Ly6G/C(Gr-1), TER119, 7-4) and therefore to eliminate the cells that are already engaged in a differentiation lineage other than the megakaryocytic one. - Following the kit instructions, resuspend the cellular pellet in freshly prepared M medium (PBS with 2% of the final volume of fetal bovine serum (FBS), EDTA 1 mM) to a concentration of 1 × 108 cells/mL and distribute the suspension in round bottomed 5 mL polystyrene tubes to a maximum volume of 2 mL.
- Add to the polystyrene tubes: normal rat serum at a concentration of 50 µL/mL as well as the biotinylated antibody mix (CD5, CD11b, CD19, CD45R/B220, Ly6G/C(Gr-1), TER119, 7-4) at a concentration of 50 µL of mix per mL and homogenize by gently flicking the tubes.
NOTE: These antibodies will bind to cells already engaged into a differentiation pathway except the megakaryocytic pathway. - Incubate the tubes on ice for 15 min.
- Add streptavidin-coated magnetic beads at a concentration of 75 µL/mL and homogenize by gently flicking the tubes.
- Incubate again on ice for 10 min.
- If necessary, adjust to a final volume of 2.5 mL per tube with M medium.
- Homogenize the suspension by gently flicking the tube just before placing them, without their caps, inside a magnet and wait for three minutes.
NOTE: The cells already engaged into a differentiation pathway and coated with magnetic beads will be retained on the wall of the tube inside the magnet. - Invert magnet and tube to transfer the tube content into a new round-bottomed 5 mL polystyrene tube.
NOTE: Do not take the tube out of the magnet for the transfer; it is done by inverting the magnet with the tube still in. Use a steady movement and do not shake the tube. - Discard the initial tube containing undesired magnetic-labeled cells and place the new one, without its cap, in the magnet for three more minutes.
- Proceeding as in step 2.20, transfer the isolated Lin− cells into a new 15 mL tube.
NOTE: If several 5 mL polystyrene tubes have been used for the previous steps, pool all the cells in the same 15 mL tube. The cells recovered after the cell sorting are hematopoietic stem cells and progenitors. The presence of thrombopoietin (TPO), the major physiological regulator of megakaryopoiesis 10, will direct the cell differentiation toward the megakaryocytic cell line. - Measure the Lin− cell number and viability as in step 2.10.
- Calculate the required volume of cell suspension to centrifuge in order to have 1 x 106 viable cells x Well Number, Well Number being the number of wells to seed per condition.
- Prepare one tube per condition with the appropriate volume of cell suspension and centrifuge at 300 x g for 7 min.
- For liquid cultures, discard the supernatant and resuspend the cell pellet in complete culture medium (DMEM, PSG 1% of the final volume, FBS 10% of the final volume, hirudin 100 U/mL, TPO 50 ng/mL) to achieve the final concentration of 2 × 106 viable cells/mL (equal to 1 × 106 cells per 500 µL well). Incubate the cells at 37 °C under 5% CO2. (= day 0 of culture)
NOTE: See the next paragraph for methylcellulose cultures As an example, to prepare complete culture medium for one well, use 435 µL of DMEM, 50 µL of 100% FBS for 10% final, 5 µL of 100% PSG for 1% final, 5 µL of 10 000 U/mL for 100 U/mL final and 5 µL of 5 µg/mL TPO for 50 ng/mL final. 4-well or 24-well culture plates are typically used as their well diameter is a good fit for the 500 µL needed per well.
3. Cell embedding in methylcellulose hydrogel
NOTE: Please note that the following protocol describes the method to obtain a single well of hydrogel cell culture, adapt to the number of wells needed.
- Thaw 1 mL aliquots of 3% methylcellulose stock solution at room temperature. Prepare one separate extra aliquot of methylcellulose for syringe coating.
NOTE: At a concentration of 3%, methylcellulose remains liquid at room temperature (20-25 °C). - Coat a 1 mL Luer lock syringe equipped with an 18-gauge needle with methylcellulose by drawing 1 mL of methylcellulose from the extra aliquot. Totally expel the methylcellulose.
NOTE: This coating step ensures that the volume of methylcellulose collected in step 3.3 is exact. - With the same syringe and needle but using a new methylcellulose aliquot, draw the appropriate volume of methylcellulose (Figure 2A).
NOTE: To achieve a final concentration of 2% methylcellulose in a final volume of 500 µL per well, 333 µL of 3% methylcellulose is required. - Cautiously remove the needle. Using sterilized forceps, screw a Luer lock connector onto the end of the syringe (Figure 2B-C).
- Attach a second, non-coated, 1 mL Luer lock syringe to the Luer lock connector in order to connect the two syringes together (Figure 2D).
NOTE: There is no need to coat this second syringe. - Equally distribute the methylcellulose volume between the two syringes (Figure 2E) and put them aside until step 3.11.
- Prepare the concentrated DMEM culture medium so as to obtain in the final methylcellulose volume (step 3.11) a concentration identical to the one of the liquid culture medium for each compound (PSG 1% of the final volume, FBS 10% of the final volume, hirudin 100 U/mL, TPO 50 ng/mL).
- Prepare 167 µL of concentrated culture medium per final well of 1 × 106 cells. This volume of medium is calculated so as to obtain a final methylcellulose concentration of 2%. The total volume in the well will be 500 µL (167 µL of cell suspension in concentrated culture medium + 333 µL of methylcellulose) and all the components will have a concentration identical to that in liquid wells.
- As an example, to prepare complete culture medium for one well, use 102 µL of DMEM, 50 µL of 100% FBS for 10% final, 5 µL of 100% PSG for 1% final, 5 µL of 10 000 U/mL for 100 U/mL final and 5 µL of 5 µg/mL TPO for 50 ng/mL final. It gives a volume of 167 µL used to resuspend the cells and with the addition of 333 µL of methylcellulose the final volume will be 500 µL.
- After completing the centrifugation step 2.26, discard the supernatant and resuspend the cell pellet in the concentrated culture medium at a ratio of 1 × 106 cells per 167 µL.
- Take back the syringes and disconnect one of them from the connector.
- Pipette 167 µL of the cell suspension.
- Add the cell suspension directly into the syringe connector (Figure 2F), making sure not to introduce air bubbles.
NOTE: While adding the cell suspension, slowly draw the syringe plunger simultaneously to free some space for the cell suspension. - Carefully reconnect the two syringes (Figure 2G) without losing any suspension in the screw thread.
NOTE: Prior to the reconnection, draw the plunger in order to leave the connector half empty and let enough space for the second syringe to connect without the suspension overflowing. - Slowly homogenize the methylcellulose medium with the cell suspension with ten back-and-forth plunger movements between the two syringes (Figure 2H).
- Draw the total volume into one syringe and disconnect the two syringes, leaving the connector on the empty one.
- Empty the content of the syringe into a well of a 4-well plate (Figure 2I).
- Incubate the cells at 37 °C under 5% CO2 (= day 0 of culture).
NOTE: It is possible to prepare two methylcellulose wells with one pair of syringes. Increase by two the volume of methylcellulose and the volume of cell suspension to have 2 x106 cells. After completing step 3.13. distribute the volume equally between the two syringes to have 500 µL in each of them. Disconnect them and empty the one without the connector in a culture well. Reconnect the syringes to transfer the volume from the one that kept the connector to the other. Disconnect the syringes, the one with the 500 µL should not have the connector attached, and seed the cells in a second culture well. The 3% methylcellulose is purchased as a stock solution in Iscove's Modified Dulbecco's Medium (IMDM) while concentrated cells are suspended in DMEM. Comparative tests have been initially done to make sure that this mixed medium had no impact on the outcome of the experiment, especially compared to the liquid culture in 100% DMEM.
4. Cell Resuspension for Proplatelet Analysis
NOTE: Analysis of the capacity to form proplatelets has to be performed under comparable conditions between liquid and methylcellulose grown megakaryocytes. The physical constraints exerted by the methylcellulose hydrogel inhibit proplatelet extension. Therefore, methylcellulose-grown cells are resuspended in fresh liquid medium on day 3 of culture to allow them to extend proplatelets. Methylcellulose hydrogel is a physical hydrogel that is easily diluted upon liquid medium addition. Importantly, to avoid artifacts from resuspension and centrifugation, cells in the control liquid medium condition have to be treated simultaneously in the same way as methylcellulose-grown cells. Refer to the schematic representation of the experiment (Figure 1).
- Prepare 10 mL of DMEM – 1% PSG preheated at 37 °C in a 15 mL tube for each well to resuspend.
- Cautiously resuspend the cells from each well in the 10 mL of DMEM – 1% PSG.
NOTE: Gently do several up-and-down movements to dilute the methylcellulose completely. For the liquid wells make sure to collect all the cells deposited at the bottom of the well. - Centrifuge the tubes 5 min at 300 × g.
- Meanwhile prepare complete culture medium (DMEM, PSG 1% of the final volume, FBS 10% of the final volume, hirudin 100 U/mL, TPO 50 ng/mL).
NOTE: At this step each well is retrieved to be diluted by half, therefore prepare 1 mL of complete culture medium per well. - Discard the supernatant and resuspend the cell pellet in 1 mL of culture medium for each tube.
- Reseed 500 µL of cell suspension per well in a 4 or 24-well plate and incubate at 37 °C under 5% CO2.
NOTE: From one initial well, obtain 2 wells for proplatelet visualization in duplicate. Please note that as these duplicates originate from the same sample they cannot be considered as independent replicates. - 24 hours after reseeding, on day 4 of culture, randomly acquire 10 images per well using bright field microscopy and the 20× objective.
NOTE: The cells tend to group at the center of the well, make sure not to have too many cells on the field as it may render proplatelet visualization and quantification difficult. Make sure to capture at least 5 megakaryocytes per field. - Count the total number of megakaryocytes and of megakaryocytes extending proplatelets in each image and calculate the proportion of megakaryocytes extending proplatelets.
NOTE: The quantification is not automated; perform cell counting manually. Counting can be facilitated by the use of the cell counter plugin of ImageJ to click on the cells in order to mark them as they are counted. 10 fields acquired per well and wells in duplicate represent approximately 150-300 megakaryocytes per condition.
5. Cell fixation and retrieval for future analyses
CAUTION: This protocol uses fixatives which must be handled under a fume hood, wearing protective equipment.
NOTE: The aim is to maintain intact the gel constraints applied on the cells until they are fully fixed. Therefore, and regardless of the fixative used, it must be added in the well on top of the methylcellulose, without disturbing the gel. The same protocol is applied to liquid cultures.
- Add a volume of fixative solution equal to the seeded volume (500 µL in this protocol), on top of the methylcellulose without disrupting the gel. Wait for the appropriate time according to the fixative used (at least 10 min).
NOTE: The fixative diffusion throughout the gel should be very rapid as revealed by a rapid change in the gel color (from pink to a yellow-orange shade). Paraformaldehyde (8% in DPBS, 500 µL per well) is usually used for immunolabeling, while glutaraldhehyde (5% in cacodylate buffer, 500 µL per well) is used for electron microscopy analysis. - Using a P1000 pipette gently do several up-and-down pipettings with the fixative and the gel so as to homogeneously dilute the methylcellulose.
- Using the same pipette and tip, transfer all the volume from the well into a 15 mL tube containing 10 mL of DPBS and homogenize.
- Centrifuge the mixture at 300 × g for 7 min.
NOTE: A second wash step might be needed to eliminate all the methylcellulose - Discard the supernatant and resuspend the megakaryocyte pellet in the appropriate medium according to the desired analysis (immunolabeling, flow cytometry9, electron microscopy …) (for electron microscopy see also the paper method "In situ exploration of the major steps of megakaryopoiesis using transmission electron microscopy" in this JoVE issue).
Representative Results
Data obtained using this protocol were originally published in Blood in 20169.
According to the protocol, the cells were seeded in either liquid or methylcellulose hydrogel medium. Cells in liquid medium have all sedimented at the bottom of the well, in contact with the stiff plastic surface and sometime with other cells. In contrast, cells embedded in methylcellulose hydrogel are distributed homogeneously in the gel and are isolated from neighboring cells (Figure 3A). Methylcellulose gel at a final concentration of 2% very slightly increases the mean megakaryocyte diameter compared to the liquid culture (Figure 3B), in accordance with the higher reported ploidy9. By contrast, increasing methylcellulose concentration by 0.5% impairs megakaryocyte differentiation as shown by a smaller mean diameter (Figure 3B).
A noticeable difference in megakaryocyte ultrastructure is observed between megakaryocytes differentiated in liquid culture and those differentiated in vivo within the bone marrow. A characteristic feature of mature megakaryocytes is a complex intracytoplasmic membrane network, the DMS (Demarcation Membrane System) which serves as a reservoir for the membrane of the future platelets. In mature megakaryocytes the DMS organizes to form intertwined membrane sheets which occupy most of the cytoplasm. By transmission electron microscopy (TEM), they appear to be closely apposed and delineate cytoplasmic territories (Figure 3C upper panel) (for the TEM procedure, see the paper method "In situ exploration of the major steps of megakaryopoiesis using transmission electron microscopy" in this same JOVE Issue). In liquid culture, DMS membranes have mostly the appearance of small round, oval, or elongated vesicles without delimitation of cytoplasmic territories (Figure 3C middle panel). By contrast, 2% methylcellulose culture promotes the organization of the DMS in a majority of megakaryocytes, with membranes closely apposed and delimiting cytoplasmic territories, resembling the one in situ (Figure 3C lower panel). This result indicates that the 2% methylcellulose hydrogel culture allows for better megakaryocyte differentiation due to the mechanical constraints of the environmental medium.
After cell transfer into liquid medium at day 3, megakaryocytes begin to extend proplatelets after 4 h 9. Figure 4 shows the quantification of the proportion of megakaryocytes having extended proplatelets 24 h after resuspension in liquid milieu. Ten images were randomly acquired per well, using bright field microscopy and the 20× objective (Figure 4A). The quantification was performed blindly and manually using the cell counter plugin on Fiji (ImageJ) (Figure 4B). Because these are primary cell cultures, there is an inter-experiment variability but the protocol remains robust and offers a good reproducibility. In the liquid pre-culture condition, the proplatelet proportion should be between 10% and 20% whereas this proportion is doubled for the hydrogel pre-culture.
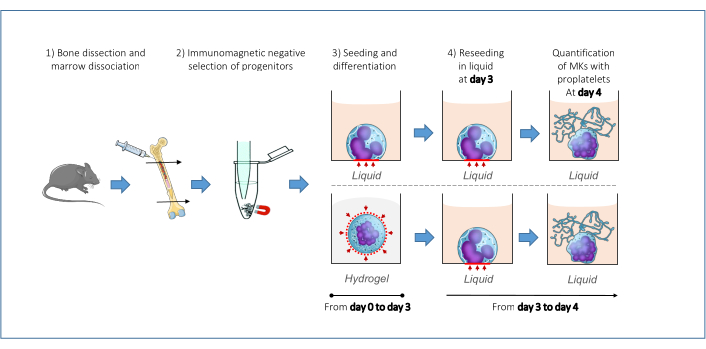
Figure 1. Schematic representation of the whole process. Bones are dissected out, marrow is flushed out and cells mechanically dissociated. Stem and progenitor cells of interest (Lin- cells) are isolated by an immunomagnetic negative sorting procedure and seeded in either liquid or hydrogel medium (day 0). At day 3 of culture (which in total represents a duration of 4 days), both conditions are resuspended in separate fresh liquid culture milieu. This second culturing step is carried out from day 3 until day 4 of culture. The proportion of MKs extending proplatelets is measured at day 4 of culture. For visual clarity, one cell is schematized per well. The blue circle is depicting a single cell with its nucleus in purple. In the final step, both MKs are represented with proplatelet. The proportion of MKs forming proplatelets varies depending on liquid or methylcellulose pre-culture. Please click here to view a larger version of this figure.
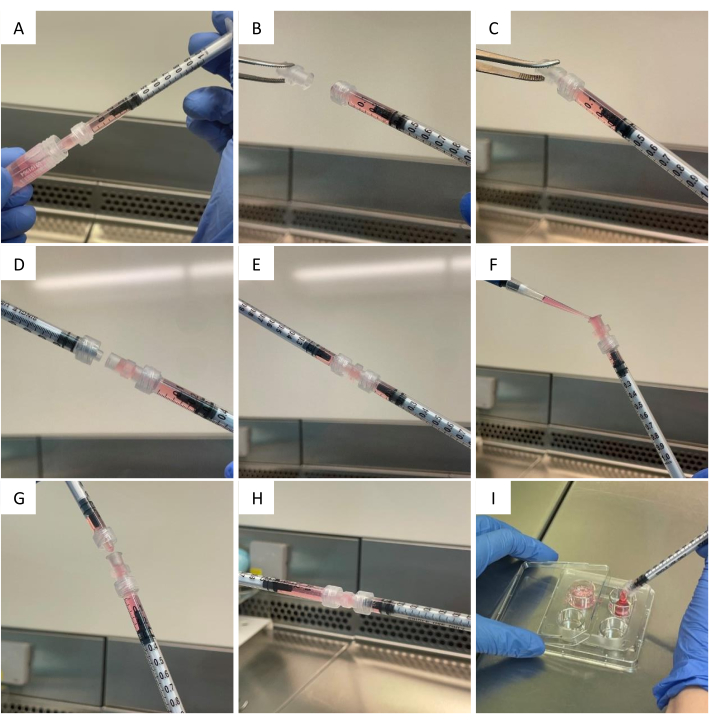
Figure 2. Cell embedding in methylcellulose hydrogel. After pre coating the syringes wall, (A) draw the appropriate volume of methylcellulose; (B, C) disconnect the needle and screw a connector onto the syringe; (D) push the methylcellulose halfway through the connector and attach a second syringe; (E) distribute equally the methylcellulose between the two syringes and disconnect them; (F) add the cell suspension to the syringe bearing the connector; (G) reconnect the two syringes; (H) homogenize by pushing the whole volume from one syringe to the other a few times; (I) seed the cells by expelling the whole volume into a culture dish. Please click here to view a larger version of this figure.
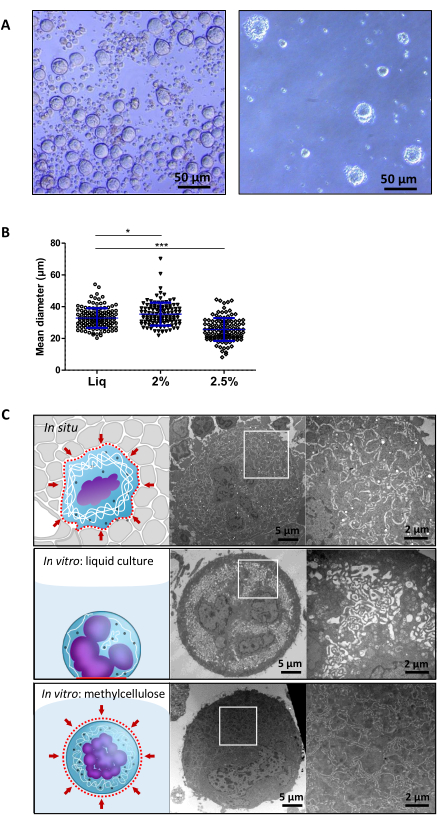
Figure 3. Megakaryocyte characteristics according to culture condition. (A) Representative images of megakaryocytes at day 3 of culture in liquid (left panel) or 2% methylcellulose hydrogel medium (right panel). Scale bar = 50 µm (B) Mean diameter of megakaryocytes grown in liquid medium, or in 2% or 2.5% methylcellulose hydrogel. Results are expressed as the mean ± SD in 3 independent cultures, with a total of at least 100 megakaryocytes examined. *, P<0.05, ***P < 0.0001, using 1-way analysis of variance (ANOVA) with Bonferroni's multiple comparison test. (graph adapted from Aguilar et al. 2016) (C) Schematic view (left) and representative electronic microscopy images (middle) of murine megakaryocytes; right panels, close up views from the white squares (scale bar = 5 µm for the middle electronic microscopy images and 2 µm for close up views). Upper panels are in situ megakaryocytes, middle represents megakaryocytes grown in vitro in liquid culture and lower panels are megakaryocytes grown in 3D methylcellulose hydrogel. These data were originally published in Blood Journal, DOI10.1007/978-1-4939-8585-2_95. Please click here to view a larger version of this figure.
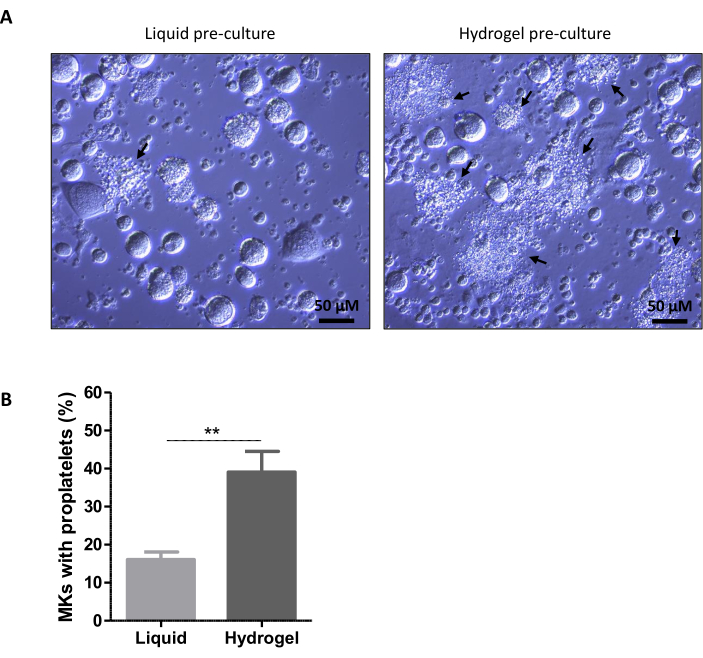
Figure 4. Representative results of proplatelet quantification. (A) representative images of megakaryocytes at day 4 of culture. Cells were incubated three days in liquid (left) or 2% methylcellulose hydrogel medium (right) followed by one day of resuspension in liquid medium. Black arrows indicate megakaryocytes extending proplatelets. (Scale bar = 50 µm). (B) Representative quantification data of proplatelet formation. Proplatelet formation quantified at day 4 for megakaryocytes previously pre-cultured from day 0 to day 3 in liquid or 2% methylcellulose hydrogel medium. Results are expressed as the% of megakaryocytes extending proplatelets (mean ± SD) and are from 3 independent experiments, with a total number of megakaryocytes examined per condition >750 (t-test, p = 0.0023). The mean proportion of megakaryocytes extending proplatelets is 16% in liquid condition and 39% for the methylcellulose hydrogel pre-culture. This result corresponds to the previously demonstrated and published effect of hydrogel pre-culture that increases proplatelet formation compared to liquid condition. Please click here to view a larger version of this figure.
Discussion
In the previous decade, mechanobiology has raised more and more interest in many areas of biology. It is now commonly acknowledged that the mechanical environment surrounding the cells does play a role in their behavior, emphasizing the importance to study how megakaryocytes sense and respond to extracellular mechanical cues. It is challenging to accurately measure the stiffness of the bone marrow tissue in situ11, especially if we consider the hematopoietic red marrow as it is located inside trabecular bones in large mammals while the more easily accessible marrow from the diaphysis is composed essentially of adipocytes (yellow marrow)12. In the case of an isolated marrow from mice, where diaphysis contains essentially red marrow, another issue is that, once extracted from the bone, the tissue does not remain cohesive. However, Shin and collaborators managed to measure mouse diaphysis marrow stiffness using atomic force microscopy and found a value of Emarrow = 0.3 ± 0.1 kPa, which places the marrow among the softest tissues6.
The interest of the procedure described here is to compare megakaryocyte behavior in liquid medium to that in the hydrogel. In liquid milieu, cells have all sedimented at the bottom of the well, in contact with the stiff plastic surface and sometime with other cells. In contrast, cells embedded in methylcellulose hydrogel are distributed homogeneously in the gel and are fully isolated from the other cells (Figure 3A). Hence they are submitted essentially to mechanical cues provided by the confinement, excluding juxtacrine communication. Paracrine stimulation cannot be totally excluded. Nonetheless, the cells embedded in the methylcellulose hydrogel are distant from one another contrary to the situation in the bone marrow and we can thus assume that if secreted substances reach neighboring cells, they might be very diluted.
The method is easy to set up and does not require specific skills. Methylcellulose is a physical gel whose polymer chains form non-covalent cross-linkages. Being liquid at low temperature, it jellifies when increasing the temperature (please see the article from Aguilar et al. 20169 for more information about the characterization of the mechanical properties of the gel). This gel state can be easily reversed following dilution in aqueous solution, which enables an easy recovery of the cells, whether fixed in gel or as live cells.
A critical factor here is the stiffness of the hydrogel. The appropriate methylcellulose volume should be very precisely dispensed as even a small change in the hydrogel concentration can have an important impact on the stiffness of the milieu and therefore on megakaryocyte maturation. For instance, it was previously shown that increasing methylcellulose concentration from 2 to 2.5% increased gel stiffness (Young's modulus) by 10 fold. One possible pitfall is that there is no easy quality control to verify precise rheological properties of the methylcellulose in each experimental well once it has been seeded with cells. Nonetheless, an essential criterion that will reassure about a correct gel concentration is the proper maturation of the megakaryocytes within the hydrogel, as reflected by their large size roughly similar to that in liquid medium. A decrease in their mean diameter could reflect a defective differentiation similar to what occurs when increasing stiffness with 2.5% methylcellulose (Figure 3B).
Another limitation of the method is that cell recovery from the hydrogel takes more time than in the classical culture as it is necessary to first dilute the gel before centrifugation. If methylcellulose needs to be totally removed, for instance to obtain cell lysate for further western blot or RNA isolation procedure, an additional washing step may be required, during which time modifications may occur in proteins or RNA (protein dephosphorylation, RNA degradation…).
A critical point to consider in the procedure is the cell count that has to be equal in each conditions. This is not that trivial since in the liquid culture, cells tend to sediment at the bottom of the well and some of them adhere on the plastic surface, which is not the case for cells in suspension in hydrogel. One pitfall is an incomplete collection of the cells in the liquid condition, resulting in a different cell content between "liquid" and "hydrogel" condition after suspension in liquid medium at day 3. Such a difference may lead to discrepancies in the final data. As a checkpoint, a cell numeration can be done at this stage before reseeding the cells. It is preferable to do it manually using a Nageotte hemocytometer as it is especially appropriate for larger cells such as megakaryocytes.
As for any primary cell culture, there is a possible risk of contamination. Contamination is the most probable explanation to an unusually low proplatelet proportion in methylcellulose pre-culture condition, as a small contamination appears more difficult to detect than in liquid medium. Therefore, it can go unnoticed until proplatelet quantification, leading to misleading results. Good laboratory practices must be strictly observed especially during methylcellulose cell encapsulation that requires numerous and precise manipulations of syringes and connectors. The megakaryocyte viability should also be checked with Trypan blue using a Nageotte cell chamber for manual counting before reseeding at day 3.
Overall, the protocol provided here describes an in vitro model for comparison between classical liquid culture and a 3D culture using methylcellulose hydrogel. Of note, this culture protocol is described for mouse primary Lin– cells and has not yet been adapted to human cells. This 3D model is a useful tool to investigate the impact of the mechanical environment on megakaryocyte behavior and maturation9. It is also possible to add compounds in the culture (even on the gel) to study the influence of drugs on megakaryocyte behavior/maturation and proplatelet formation. Finally, by reproducing the mechanical constraints that cells may encounter in the bone marrow, this culture system allows for the investigation of abnormal phenotypes that could not be observed in classical liquid cultures as previously showed for Myh9 knockout megakaryocytes9,13,14.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Fabien Pertuy and Alicia Aguilar who initially developed this technique in the lab, as well as Dominique Collin (Institut Charles Sadron – Strasbourg) who characterized the viscoelastic properties of the methylcellulose hydrogel. This work was supported by ARMESA (Association de Recherche et Développement en Médecine et Santé Publique) and by an ARN grant (ANR-18-CE14-0037 PlatForMechanics). Julie Boscher is a recipient from the Fondation pour la Recherche Médicale (FRM grant number FDT202012010422).
Materials
| 18-gauge needles | Sigma-Aldrich | 1001735825 | |
| 21-gauge needles | BD Microlance | 301155 | |
| 23-gauge needles | Terumo | AN*2332R1 | |
| 25-gauge neeldes | BD Microlance | 300400 | |
| 4-well culture dishes | Thermo Scientific | 144444 | |
| 5 mL syringes | Terumo | SS+05S1 | |
| Cytoclips | Microm Microtech | F/CLIPSH | |
| Cytofunnels equiped with filter cards | Microm Microtech | F/JC304 | |
| Cytospin centrifuge | Thermo Scientific | Cytospin 4 | |
| Dakopen | Dako | ||
| DMEM 1x | Gibco, Life Technologies | 41 966-029 | |
| DPBS | Life Technologies | 14190-094 | Sterile Dulbecco’s phosphate-buffered saline |
| EasySep magnets | Stem Cell Technologies | 18000 | |
| EasySep Mouse Hematopoietic Progenitor Cell isolation Kit | Stem Cell Technologies | 19856A | biotinylated antibodies (CD5,CD11b, CD19, CD45R/B220, Ly6G/C(Gr-1), TER119,7–4) and streptavidin-coated magnetic beads |
| EDTA | Invitrogen | 15575-020 | |
| Fetal Bovine Serum | Healthcare Life Science | SH30071.01 | |
| Luer lock 1 mL syringes | Sigma-Aldrich | Z551546-100EA | or 309628 syringes from BD MEDICAL |
| Luer lock syringes connectors | Fisher Scientific | 11891120 | |
| MC 3% | R&D systems | HSC001 | |
| Polylysin coated slides | Thermo Scientific | J2800AMNZ | |
| PSG 100x | Gibco, Life Technologies | 1037-016 | 10,000 units/mL penicillin, 10,000 μg/mL streptomycin and 29.2 mg/mL glutamine |
| Rat serum | Stem Cell Technologies | 13551 | |
| Recombinant hirudin | Transgène | rHV2-Lys47 | |
| Recombinant human trombopoietin (rhTPO) | Stem Cell Technologies | 2822 | 10,000 units/mL |
| Round bottomed 10 mL plastique tubes | Falcon | 352054 | |
| Round bottomed 5 mL polystyrene tubes |
References
- Doolin, M. T., Moriarty, R. A., Stroka, K. M. Mechanosensing of Mechanical Confinement by Mesenchymal-Like Cells. Frontiers in Physiology. 11, (2020).
- Wang, C., et al. Matrix Stiffness Modulates Patient-Derived Glioblastoma Cell Fates in Three-Dimensional Hydrogels. Tissue Engineering Part A. , (2020).
- Doyle, A. D., Yamada, K. M. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Experimental Cell Research. 343 (1), 60-66 (2016).
- Engler, A. J., Sen, S., Sweeney, H. L., Discher, D. E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 126 (4), 677-689 (2006).
- Choi, J. S., Harley, B. A. C. The combined influence of substrate elasticity and ligand density on the viability and biophysical properties of hematopoietic stem and progenitor cells. Biomaterials. 33 (18), 4460-4468 (2012).
- Shin, J. -. W., et al. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell stem cell. 14 (1), 81-93 (2014).
- Boscher, J., Guinard, I., Eckly, A., Lanza, F., Léon, C. Blood platelet formation at a glance. Journal of cell science. 133 (20), (2020).
- Aguilar, A., Boscher, J., Pertuy, F., Gachet, C., Léon, C. Three-dimensional culture in a methylcellulose-based hydrogel to study the impact of stiffness on megakaryocyte differentiation. Methods in Molecular Biology. 1812, 139-153 (2018).
- Aguilar, A., et al. Importance of environmental stiffness for megakaryocyte differentiation and proplatelet formation. Blood. 128, 2022-2032 (2016).
- Hitchcock, I. S., Kaushansky, K. Thrombopoietin from beginning to end. British Journal of Haematology. 165 (2), 259-268 (2014).
- Leiva, O., Leon, C., Kah Ng, S., Mangin, P., Gachet, C., Ravid, K. The role of extracellular matrix stiffness in megakaryocyte and platelet development and function. American Journal of Hematology. 93 (3), 430-441 (2018).
- Jansen, L. E., Birch, N. P., Schiffman, J. D., Crosby, A. J., Peyton, S. R. Mechanics of intact bone marrow. Journal of the Mechanical Behavior of Biomedical Materials. 50, 299-307 (2015).
- Eckly, A., et al. Abnormal megakaryocyte morphology and proplatelet formation in mice with megakaryocyte-restricted MYH9 inactivation. Blood. 113 (14), 3182-3189 (2009).
- Eckly, A., et al. Proplatelet formation deficit and megakaryocyte death contribute to thrombocytopenia in Myh9 knockout mice. Journal of Thrombosis and Haemostasis. 8 (10), 2243-2251 (2010).

