Measurement of Protein Turnover Rates in Senescent and Non-Dividing Cultured Cells with Metabolic Labeling and Mass Spectrometry
Summary
This protocol describes the workflow for metabolic labeling of senescent and non-dividing cells with pulsed SILAC, untargeted mass spectrometry analysis, and a streamlined calculation of protein half-lives.
Abstract
Mounting evidence has shown that the accumulation of senescent cells in the central nervous system contributes to neurodegenerative disorders such as Alzheimer's and Parkinson's diseases. Cellular senescence is a state of permanent cell cycle arrest that typically occurs in response to exposure to sub-lethal stresses. However, like other non-dividing cells, senescent cells remain metabolically active and carry out many functions that require unique transcriptional and translational demands and widespread changes in the intracellular and secreted proteomes. Understanding how protein synthesis and decay rates change during senescence can illuminate the underlying mechanisms of cellular senescence and find potential therapeutic avenues for diseases exacerbated by senescent cells. This paper describes a method for proteome-scale evaluation of protein half-lives in non-dividing cells using pulsed stable isotope labeling by amino acids in cell culture (pSILAC) in combination with mass spectrometry. pSILAC involves metabolic labeling of cells with stable heavy isotope-containing versions of amino acids. Coupled with modern mass spectrometry approaches, pSILAC enables the measurement of protein turnover of hundreds or thousands of proteins in complex mixtures. After metabolic labeling, the turnover dynamics of proteins can be determined based on the relative enrichment of heavy isotopes in peptides detected by mass spectrometry. In this protocol, a workflow is described for the generation of senescent fibroblast cultures and similarly arrested quiescent fibroblasts, as well as a simplified, single-time point pSILAC labeling time-course that maximizes coverage of anticipated protein turnover rates. Further, a pipeline is presented for the analysis of pSILAC mass spectrometry data and user-friendly calculation of protein degradation rates using spreadsheets. The application of this protocol can be extended beyond senescent cells to any non-dividing cultured cells such as neurons.
Introduction
Senescence was first identified as a state of indefinite growth arrest exhibited by cultured primary cells after reaching replicative exhaustion1. It has since been shown that senescence can arise in response to numerous cellular insults, including genotoxic, mitochondrial, and oncogenic stresses, among others2. While senescence has several physiologically important roles, such as tumor suppression and wound healing, the accumulation of senescent cells during aging is associated with a host of deleterious effects on health3, including several neurodegenerative conditions4,5,6. Cellular senescence occurs in multiple brain cell types, including neurons7,8,9,10, astrocytes11, microglia12, and oligodendrocyte precursors13, and contributes to neurodegeneration and cognitive dysfunction. Amyloid beta oligomers, one of the hallmarks of Alzheimer's disease14, have been shown to accelerate neuronal senescence13,15,16. An increased prevalence of senescent cells has also been associated with Parkinson's disease17, especially arising from environmental stressors11,18. Importantly, the selective elimination of senescent cells in pre-clinical models extends lifespan and mitigates a multitude of age-related diseases3,5,12 and improves cognitive deficits8,11,12,13. Senescent cells have thus emerged as promising therapeutic targets for the treatment of many age-related conditions.
Much of the detrimental effect of senescent cells is caused by the senescence-associated secretory phenotype (SASP), a complex mixture of bioactive molecules secreted by senescent cells that can cause local inflammation, angiogenesis, destruction of the extracellular matrix, and propagation of senescence in surrounding tissue19,20,21. The SASP also represents an interesting biological phenomenon of senescence because it requires a considerable transcriptional and translational effort during a state of cell cycle arrest. In fact, senescent cells have been shown to exhibit decreases in ribosome biogenesis22,23,24 that should reduce protein synthesis. Instead, senescent cells robustly translate some proteins, particularly SASP factors, and influence the metabolism of surrounding tissue25. Thus, there is considerable interest in understanding how senescent cells undergoing a permanent cell cycle arrest continue to maintain protein homeostasis while at the same time robustly express SASP factors and other select proteins.
This method describes how to use mass spectrometry and pulsed-stable isotope labeling by amino acids in cell culture (pSILAC) to globally measure the half-lives of proteins in senescent cells at a proteome-wide scale. In traditional SILAC, cultured cells are completely metabolically labeled with heavy and light non-radioactive isotopes of amino acids for downstream analysis of protein abundance. This method has been previously applied to assess abundance changes comprehensively and quantitatively in the SASP of cultured fibroblasts26. In pSILAC, cells are similarly metabolically labeled with a pulse of heavy isotope that follows pre-labeling with light isotope, and then harvested at one or more time intervals. The rates of incorporation of heavy isotope in reference to pre-existing light isotope are then used to calculate relative protein turnover rates. Generally, isotopes of arginine and lysine are used because trypsin cleaves at those residues; thus, all peptides from standard digestion will potentially contain the heavy label. Pairs of peptides that differ only by the presence or absence of heavy lysine or arginine are chemically identical and can be differentiated and quantified by a mass spectrometer. Following mass spectrometry analysis, peptides can be identified as newly synthesized or pre-existing based on the presence or absence of the isotopic label in the resulting peptide identifications. Protein turnover rates can then be determined by fitting the ratio of heavy (13C-15N) over light (12C-14N) peptides for a given protein to kinetic models for exponential growth or decay27,28. pSILAC has been used in several comparisons of protein turnover rates29,30,31,32 and is currently the most comprehensive and high-throughput method for the measurement of protein half-lives.
This protocol details the preparation of senescent cells in parallel with similarly growth-arrested quiescent cells in culture, followed by metabolic labeling with pSILAC. Cells are then harvested, homogenized into lysates, and processed for mass spectrometry acquisition and analysis. The data obtained from mass spectrometry are then used to determine protein half-lives using a simplified quantitative method employing a single time point and half-life calculations performed in a spreadsheet. Using this approach, estimates of protein half-lives can be measured in a comprehensive and quantitative manner that is more authentic to unperturbed cellular conditions than protocols that use blockers of protein synthesis or turnover.
Protocol
1. Preparation of quiescent cells and cells rendered senescent by exposure to ionizing radiation (IR)
NOTE: Cellular senescence and quiescence can be induced using multiple methods, as described in detail elsewhere33,34,35. The stimuli used to induce senescence and quiescence may depend on the cell type of interest and the biological question under investigation. The cells used in this study are commercially available.
- Thaw human diploid IMR-90 fibroblasts (~1 x 106 cells) from a cryovial and plate them in 20 mL of DMEM supplemented with 10% Fetal Bovine Serum (FBS) (Table 1) on a 150 mm plate.
- Grow the cells at physiological oxygen conditions (3% O2, 5% CO2, 37 °C) and expand the cultures in 10% FBS-containing media until sufficient replicates for quiscent and senescent cells have been established (at least 3-5 replicates are recommended per condition).
NOTE: Culturing cells at physiological (3%) oxygen is ideal for primary human fibroblast cells, but suitable culture conditions for other cell types may vary and should be determined on a case-by-case basis. - Generate senescent cells by exposing proliferating cells to 15 gray (Gy) of ionizing radiation (IR).
NOTE: Intensity of radiation exposure may vary according to cell type. While 15 Gy is used here, 10 Gy is also a commonly used radiation dose for fibroblasts; other cells, such as monocytes, may require a dose as low as 5 Gy. The dose is generally determined empirically by weighing viability against senescence induction.- Expose the cells at 40%-60% confluence to IR in media containing 10% FBS.
- After IR exposure, change to fresh media containing 10% FBS.
- Change media (20 mL, containing 10% FBS) every 2 days for 8 days.
NOTE: Cells are exposed to IR at lower confluency because IR-treated cells will expand in culture before they cease growing. In this experiment, cells were not split cells during the establishment of senescence, and while they became more confluent, they still displayed senescent cell markers at harvest. In fibroblasts, the senescent phenotype develops within 7-10 days.
- Generate quiescent control cells by changing the media on plated proliferating cells to media containing 0.2% FBS (serum starvation) (Table 1).
- Continue growing cells that will be used for the quiescence control in media containing 10% FBS until day 4 after senescent cells are exposed to IR, splitting when necessary.
- On day 4 after IR, change the media of quiescent cells to 20 mL of media containing 0.2% FBS (Table 1) and continue growing for another 6 days, changing media every 2 days.
NOTE: Even in media containing 0.2% FBS, fibroblasts will continue to grow somewhat and may appear confluent by the end of the harvest. As a rule of thumb, aim to collect quiescent cells when they have reached confluency similar to that of the senescent cell cultures.
2. Labeling of cells for pulsed SILAC and harvest of lysates
- Change media on both senescent and quiescent cells (12 plates) to SILAC Light (Table 1) and grow for 2 days.
NOTE: This labeling helps reduce background noise since there is a low natural abundance of 13C and 15N. This step can be skipped in reagent-limiting conditions but will result in a slight overestimation of new protein synthesis. - Replace media with SILAC DMEM (Table 1) for metabolic labeling.
NOTE: SILAC DMEM media are specially formulated to contain purely light or heavy isotopes of arginine and lysine for metabolic labeling. They must not be substituted with standard DMEM formulations in substeps 2.2.1 or 2.2.2.- For three senescent and three quiescent plates, replace media with 30 mL of SILAC Light (Table 1) and grow for 3 days without changing media.
- For a minimum of three senescent and three quiescent plates, replace the media with 30 mL of SILAC Heavy (Table 1) and grow for 3 days without changing media.
NOTE: There is an option at this point to harvest what will be the light-labeled cells immediately, rather than label them for an additional 3 days. For a single time point, it is preferable to label heavy and light-labeled cells for the same period as is done in this protocol to minimize batch effects.
- Detach the cells from culture plates by adding 5 mL of pre-warmed trypsin reagent to each dish and incubating for 5 min at 37 °C.
- Resuspend the detached cells in 5 mL of the same media used for culture (either SILAC Light or SILAC Heavy) to a total volume of 10 mL.
- Harvest the cells for extraction of lysates and validation of senescence markers.
- For each suspension, aliquot 0.6 x 106 of cells into 2 mL of media containing 0.2% FBS in a 6-well dish (two dishes, one for SILAC Light cultured cells and one for SILAC Heavy cultured cells) and place at 37 °C for overnight incubation.
NOTE: These plates (substep 2.5.1) will be used for the detection of senescence-associated β-galactosidase (SA-βGal) activity (step 3.1). - For each suspension, aliquot 1 x 106 of cells into a microcentrifuge tube and spin down in a tabletop centrifuge at full speed for 1 min. Remove the supernatant and resuspend the pellet in 1 mL of phenol; at this step, RNA can be fully purified as described in the phenol supplier's manual or stored long-term at -80 °C.
CAUTION: Phenol is corrosive and should be handled exclusively in a hood with gloves and a lab coat.
NOTE: The extracted RNA will be used for the detection of mRNAs encoding SASP factors using reverse transcription (RT) followed by real-time, quantitative (q) PCR (RT-qPCR) analysis (step 3.2). Another reliable assay for senescence induction is a test for 5-ethynyl dihydroxy uridine (EdU) incorporation, which indicates the presence of proliferating cells. The absence of proliferation can be used to confirm quiescence and senescence. - Transfer the remaining cells to ice and spin down at 300 x g and 4 °C.
- For each suspension, aliquot 0.6 x 106 of cells into 2 mL of media containing 0.2% FBS in a 6-well dish (two dishes, one for SILAC Light cultured cells and one for SILAC Heavy cultured cells) and place at 37 °C for overnight incubation.
- Remove the supernatant and wash the cells twice in 1 mL of cold PBS to remove media/trypsin and exogenous protein contamination from fetal bovine serum from the culture medium.
- Spin down the cells again, remove the supernatant, and proceed to lysis.
- Resuspend the cell pellets in 150 µL of freshly prepared 8 M urea 50 mM ammonium bicarbonate Lysis Buffer (Table 1), and mix by pipetting up and down.
- Sonicate the lysates in a water sonicator for 2.5 min with 30 s on/off at medium power at 4 °C.
- Transfer the lysates to a pre-heated 95 °C heat block and denature for 4 min.
- Spin down the lysates; lysates can now be stored at -80 °C or used immediately for quantification.
- Make a 30 µL stock of 1:10 dilutions for each lysate in Lysis Buffer.
- Measure the protein concentration with the BCA Assay Kit using a standard curve prepared in 1/10x Lysis Buffer diluted in ddH2O. Lysates can now be stored at -80 °C indefinitely.
3. Validation of senescence by senescence-associated β-Galactosidase (SA-βGal) activity and RT-qPCR analysis of senescence-associated mRNAs
NOTE: The starting material for these steps is collected during step 2.5
- Starting from the 6-well plates that were set up in substep 2.5.1, analyze cells for SA-βGal activity using the Senescence β-Galactosidase Staining kit following the manufacturer's protocol. Visualize staining under brightfield with color enabled, as previously described36.
NOTE: SA-βGal is quantified by comparing the percentage of positive cells (visible blue color) in senescent versus quiescent control conditions. A minimum of 70% of cells should be SA-βGal positive for successful confirmation of senescence. Quiescent control cells should be less than 10% positive for SA-βGal. - Extract the RNA from phenol suspension (substep 2.5.2) and analyze using RT-qPCR for increased levels of mRNAs encoding SASP factors (IL6, CXCL8, IL1B), cell cycle markers (CDKN2A/p16, CDKN1A/p21), and other indicators of senescence (loss of LMNB1 and PCNA).
NOTE: RNA is unstable and should therefore be handled with RNase-free equipment, fresh gloves, and on ice unless otherwise noted in the protocol.- Starting from phenol suspension, add 200 µL of chloroform per 1 mL of phenol and spin down at 12,000 x g for 15 min at 4 °C.
- Carefully remove the aqueous phase and add 1:1 volume of isopropanol, 15 µg of glycogen co-precipitant, and then incubate at 4 °C for 10 min to precipitate RNA.
- After incubation, pellet the RNA by centrifugation at 12,000 x g for 20 min at 4 °C followed by a wash in 75% EtOH equal to 1 volume of phenol used. Resuspend the RNA in 50 µL of nuclease-free distilled water; RNA can be stored at -80 °C indefinitely.
- To RNA samples, add 38 µL of nuclease-free distilled water, 10 µL of 10x DNAse reaction buffer, and 2 µL of DNase I followed by mixing.
NOTE: Generating a common mix of all the reagents in substep 3.2.3 multiplied by the number of samples for treatment for equal distribution to samples is the best practice. - Incubate DNase I-treated RNA samples at 37 °C for 30 min.
- Remove DNase from the samples using 1 volume of a phenol/chloroform/isoamyl alcohol (25:24:1) mixture by vortexing and spinning at max speed in a tabletop centrifuge for 5 min; the RNA will be contained in the aqueous phase.
- Repeat substeps 3.2.2 and 3.2.3 to precipitate RNA. Resuspend in 20 µL of nuclease-free distilled water; RNA can be stored at -80 °C indefinitely.
- Generate cDNA from purified RNA by incubating 0.5-1.0 µg of purified RNA with 200 U of reverse transcriptase, 100 pMol random primers, and 10 mM of a dNTP mix in 1x reaction buffer supplied with the reverse transcriptase; incubate for 10 min at 25 °C, then for 30 min at 50 °C, with a final inactivation step at 85 °C for 5 min.
- Analyze cDNA from the RT step (substep 3.2.3) from quiescent control and senescent cells using real-time, quantitative (q) PCR analysis to assess the level of mRNA markers known to be increased (CDKN2A/p16, CDKN1A/p21, IL1B, IL6, and CXCL8 mRNAs) or decreased (LMNB1 and PCNA mRNAs) with senescence as described elsewhere. ACTB mRNA, encoding the housekeeping protein β-Actin, is often a good mRNA to normalize differences in the input material. The primers used are in Table 2.
NOTE: Relative RT-qPCR analysis is dependent on assumptions of equal binding efficiency between the target primer pairs and a reference primer pair. These assumptions should be tested for new primer sets as detailed elsewhere37. Additionally, reference markers should be constant between the cell types being compared, and several additional options for senescent cells have been previously identified38.
4. In-solution trypsin digestion
NOTE: From this point onward, it is critical to use mass spectrometry-grade buffers, solvents, and chemicals to prevent interference from impurities during mass spectrometry analysis. All buffers should be composed of ingredients suited to liquid chromatography tandem mass spectrometry analysis, including water and acetonitrile. Refer to the Table of Materials for a list of suitable chemicals and solvents.
- Aliquot 50 µg of each protein sample into new tubes and bring to equal volumes with Lysis Buffer.
- To each sample, add DTT to a final concentration of 20 mM to reduce disulfide bonds.
- Incubate the samples at 37 °C for 30 min with shaking, and then allow the samples to cool at room temperature (RT, ~10 min).
- Add iodoacetamide to a final concentration of 40 mM to irreversibly alkylate the sulfhydryl groups that were reduced in the previous step. Incubate the samples at RT in the dark for 30 min.
- Dilute each sample to below 1 M Urea with a buffer composed of 50 mM ammonium bicarbonate. Check whether the pH is approximately 8 by pipetting a small amount of sample on pH strips.
- Add 1 µg of trypsin to each sample for 50 µg of starting protein, or at 1:50 trypsin:protein ratio, by mass, if digesting a different protein quantity. For example, 3 µg of trypsin would be added for the digestion of 150 µg of protein.
- Incubate the samples overnight at 37 °C with shaking to digest proteins into peptides.
- Add formic acid to 1%, by volume, of each sample to quench the protein digestion.
NOTE: The experiment can be paused here. Freeze the samples at -80 °C and continue to the next step at a later date if necessary.
5. Sample clean-up with solid-phase extraction (SPE)
NOTE: This solid-phase extraction protocol requires solid-phase extraction cartridges and a vacuum manifold setup. Other equivalent solid-phase extraction (SPE) protocols can be carried out at the researcher's discretion prior to mass spectrometry analysis.
- Prepare for SPE by placing solid-phase extraction cartridges on a vacuum manifold, using one extraction cartridge for each sample.
NOTE: Refer to the manufacturer's guidelines for the amount of sorbent to be used in the SPE protocol. For 50 µg of peptide samples, it is recommended to use 10 mg sorbent cartridges. - Condition each SPE cartridge by adding 800 µL of SPE Elution Buffer (Table 1) and use vacuum suction to draw the solvent through the cartridge.
- Repeat step 5.2.
- Equilibrate each cartridge by adding 800 µL of SPE Wash Buffer (Table 1) and use vacuum suction to draw the buffer through the cartridges.
- Repeat step 5.4 two additional times for a total of three times.
- Load the peptide samples into SPE cartridges and use vacuum suction to draw samples through the cartridges.
NOTE: At this point, peptides are bound to the sorbent inside the cartridges. - Wash each cartridge with SPE Wash Buffer and use vacuum suction to draw the buffer through the cartridges.
- Repeat step 5.7 two additional times for a total of three washes.
- Prior to the elution step, arrange collection tubes within the vacuum manifold below each cartridge, carefully ensuring alignment between the collection tubes and cartridges.
- To elute peptides, add 800 µL of SPE Elution Buffer to each cartridge and elute peptides into collection tubes with vacuum suction.
- Repeat step 5.10 with 400 µL of SPE Elution Buffer.
- Remove the peptide samples from the vacuum manifold and dry completely in a vacuum concentrator (drying takes approximately 3 h).
NOTE: The experiment can be paused here. Freeze samples at -80 °C and continue at a later date if necessary.
6. Data-dependent acquisition (DDA) mass spectrometry analysis
- Resuspend the peptide samples at a concentration of 400 ng/µL in a buffer composed of 0.2% formic acid in water.
- To aid in re-solubilization of peptides, vortex the samples for 5 min. Then, sonicate the samples for 5 min in a water bath sonicator.
- Pellet any insoluble materials by centrifuging samples at 15,000 x g for 15 min at 4 °C. Transfer the peptide supernatants into MS vials.
- Add indexed retention time (iRT) peptide standards of choice to each sample at a concentration of 1:30 iRT:sample, by volume.
- Submit the samples for proteomic analysis using liquid-chromatography tandem mass spectrometry (LC-MS/MS) analysis.
- Use LC-MS/MS settings recommended for untargeted analysis by the mass spectrometry facility. Example settings for analysis are shown in Figure 3, configured for analysis on an Orbitrap mass spectrometer coupled to a nano liquid chromatography system in nano-flow mode. An example protocol follows.
- Load 1 µg (5 µL) of each sample onto a trap column (1 cm long x 100 µm diameter) and wash them with Loading Solvent (Table 1) at a flow rate of 10 µL/min for 5 min.
- Load the samples onto an analytical column (50 cm long x 100 µm diameter) with a 400 nL/min flow rate.
- Elute the peptides over a 90 min linear gradient with an organic solvent (0.2 % formic acid and 99.8% acetonitrile) and inorganic solvent (0.2% formic acid in 99.8% water), ranging from 5% to 35% organic solvent.
- Acquire mass spectrometry data in data-dependent mode with a continuous cycle of MS1 survey scans (60,000 resolution, 3e6 AGC target, 100 ms maximum accumulation time, and 400-1,600 m/z mass range) followed by 20 data-dependent MS2 scans (15,000 resolution, 1e5 AGC target, 25 ms maximum injection time, and 1.6 m/z width isolation windows) with HCD fragmentation (normalized collision energy of 27%).
- Following MS acquisition of all samples, import raw mass spectrometry files into a mass spectrometry proteomics analysis software tool for identification and quantification of peptide peak areas.
NOTE: For the identification of peptides and proteins in this experiment, the Mascot database searching tool was used with the reviewed UniProt human proteome sequence database (Proteome ID: UP000005640). The following search parameters were specified in Mascot:- Quantitation: SILAC K+8 R+10 [MD] Enzyme: Trypsin/p Fixed modification: carbamidomethyl (C)
- Variable modifications: acetyl (Protein N-term), Gln->pyro-Glu (N-term Q), Oxidation (M), Label:13C(6)15N(2) (K), Label:13C(6)15N(4) (R)
- Peptide mass tolerance: 10 ppm
- Fragment mass tolerance: 0.08 Da
- Max missed cleavages: 2
- All the unspecified parameters were default
- Quantify the peptide peak areas for heavy and light peptides in proteome quantitation software tool. For the quantification of peak areas in this experiment, the free and open-source Skyline software platform was used39,40. Export heavy and light peptide peak areas for estimation of protein half-lives.
7. Calculation of protein half-lives
- Open the SILAC Analysis Workbook (Table 3) to the first sheet named 1) Raw Data and paste in UniProt IDs, gene names, heavy peak areas, and light peak areas into the indicated columns (SH = Senescent-Heavy, SL = Senescent-Light, CH = Control (quiescent)-Heavy, CL = Control (quiescent)-Light).
- Open sheets 2-4 and ensure that the UniProt ID and Gene columns match the number of proteins identified from the analysis (these experiments identified 841 proteins). The rest of the columns will automatically fill with data after they have been dragged to cover the identified proteins.
- Open the fourth sheet named 4) Analysis and remove rows where columns G and H indicate that the sample is out of range; keep rows that read within range. The volcano plot in the fifth sheet will automatically populate.
Representative Results
This protocol describes a method to globally compare protein half-lives between senescent and non-dividing, quiescent control cells using pSILAC and minimal time points. This protocol details generation of senescent and quiescent cells in culture, metabolic labeling of cells with stable isotopes of arginine and lysine over 3 days, quantifying the relative abundances of heavy and light peptide isotopes by mass spectrometry, and a straightforward and accessible calculation of protein half-lives using spreadsheet formulas (Figure 1). This method is highly flexible and can be adapted to numerous cell types and conditions.
As part of the generation of senescent cells for this protocol, two methods of senescence validation are used: SA-βGal-positive cells visualized by microscopy and increased levels of senescent markers quantified using RT-qPCR analysis. The measurement of senescent markers should yield clear distinctions between quiescent and senescent cells for comparisons of the two cells populations to be considered valid. For SA-βGal activity, senescent cells should appear blue while quiescent control cells have no or very little color (Figure 2A). This assay can be quantified by counting the positive, blue-stained cells as a percentage of the total number of cells, and then comparing the percentage positivity rate between quiescent control and senescent cells. It is important that this protocol be performed at the same time for the comparison of both cell states; SA-βGal activity relies on the pH of the staining solution, so the results can vary substantially between assays and should be considered a qualitative measure.
The RT-qPCR analysis of senescent markers will show high levels of the mRNAs encoding SASP factors (IL6, CXCL8, IL1B) and the cell cycle inhibitors (CDKN2A/p16, CDKN1A/p21) in most senescent models. Although some variation is expected based on cell type, culture condition, and senescent inducer41,42, most senescent cells display greater than five-fold higher levels of IL6, CXCL8, and CDKN1A/p21 mRNAs compared to quiescent control cells; conversely, the levels of LMNB1 and PCNA mRNAs, encoding proliferation markers, should be low or absent in senescent cells compared with quiescent cells (Figure 2B). Taken together, a significant percentage of SA-βGal positivity and the expected expression of a panel of senescence-associated mRNA markers are sufficient to confirm the induction of senescence in an experiment.
Processing protein samples for mass spectrometry analysis consists of in-solution digestion (2 days) and solid-phase extraction (4-6 h). To perform untargeted proteomic analysis, the resulting peptides are submitted for LC-MS/MS analysis using data-dependent acquisition (DDA). On Orbitrap instruments, a DDA method can be specified in the mass spectrometry instrument software method editor. The mass spectrometer settings used in this study are shown in Figure 3A. Liquid chromatography settings can also be specified within the method editor. For this study, a 90 min linear gradient with increasing organic phase (acetonitrile) was used (Figure 3B). Following a successful acquisition, the total ion current (TIC) should contain an intense signal during the linear gradient portion of the method (Figure 3C). This protocol describes an example protocol on an Orbitrap instrument, but the calculation of protein half-lives can be performed on data obtained from any mass spectrometer that was collected in DDA-mode, which is available on several types of instruments (e.g., Orbitraps and time-of-flight instruments) and vendors. The acquisition settings will be unique to the type and configuration of the instrument used, and it is recommended to use the settings suggested by the mass spectrometry facility. This method is also compatible with non-DDA methods (SRM, PRM, DIA), as long as quantitative chromatographic peak areas for heavy and light peaks can be extracted from the raw data files and entered into the spreadsheet formulas.
Raw mass spectrometry files are searched with one of the many available proteomic database search tools to identify peptides and proteins. For example, this study utilized the Mascot43 search engine. To obtain chromatographic peak areas for quantification of heavy and light peptides, database search results are imported into a proteomic software capable of chromatographic peak areas such as Skyline39,40. Examination of the extracted ion chromatograms of peptides (Figure 4) will reveal the relative proportion of heavy and light peptide signals. A lower proportion of heavy peptide signal relative to light peptide signal in senescent cells indicates a slower protein turnover rate (Figure 4A), and a higher heavy peptide signal relative to light indicates a faster protein turnover rate (Figure 4B). The unlabeled samples should show little or no heavy peptide signal. Any apparent heavy peptide signal in the unlabeled samples is considered background noise and will be subtracted during the final calculations. Quantification of chromatographic peak areas for light and heavy peptides for all treatment and labeling conditions must be exported for subsequent calculation of protein turnover rates and statistical analysis.
By using single time point analysis, the quantification of protein half-lives is simple to perform and can be done conveniently in spreadsheets. In Table 3, the half-lives for 695 proteins identified from the mass spectrometry analysis were calculated. Starting from protein-level heavy and light isotope abundances for each protein in the samples (input for the 1) Raw Data sheet), a percent heavy isotope (termed the Ratio, R) is then automatically calculated on the sheet titled 2) Ratio H | H + L. At this step, the R from heavy labeled samples is normalized by subtracting the R from unlabeled samples to produce a final R for the quiescent and senescent triplicates. Since unlabeled samples should not have any exogenously added heavy isotope, they are used to eliminate the background signal. From R, the kdeg (turnover rate) is calculated using the following formula:
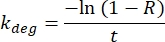
The half-life (H, in days) is determined for each protein in the quiescent control and senescent triplicates (sheet titled 3) Half-Life (days)) using the following formula:
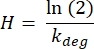
These half-lives are then averaged among the quiescent and senescent triplicates to generate a quiescent and senescent mean half-life for each protein as well as a p-value. Calculated half-lives are then filtered for values that are negative, which occurs when the heavy signal from light-labeled cells (the background) is greater than the heavy signal from heavy-labeled cells. This filtering resulted in 707 proteins from 841 identified with valid half-lives for the results presented here.
The half-life is then reported as a log2 ratio of senescent over quiescent control cells (log2FC) and plotted in a volcano plot (Figure 5). For example, by looking at the 5) Analysis sheet, the coagulation II thrombin receptor (F2R) protein can be seen to have a half-life in quiescent cells of 0.51 days (column B) and a half-life in senescent cells of 1.07 days (column C) that yielded a log2FC of ~1.06 (column D) with a p-value of 0.001.
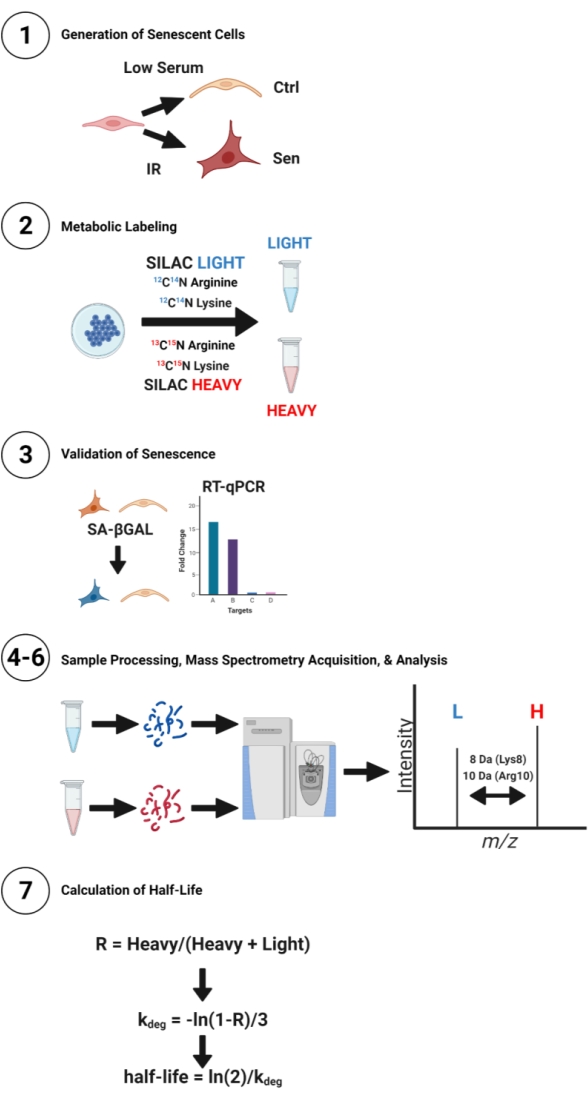
Figure 1: Diagram of pSILAC workflow for senescent and quiescent control cells. Human IMR-90 fibroblasts were used to prepare quiescent (low-serum) or senescent (IR) cell cultures for comparison of protein half-lives. Cells were then labeled with SILAC Light or SILAC Heavy media with isotopic arginine and lysine for 3 days. Lysates were extracted from cells, digested, desalted, and analyzed using mass spectrometry. Heavy and light peptide isotope peaks correspond to newly synthesized and pre-existing peptides, respectively. Half-lives were calculated using an equation for exponential decay. Please click here to view a larger version of this figure.
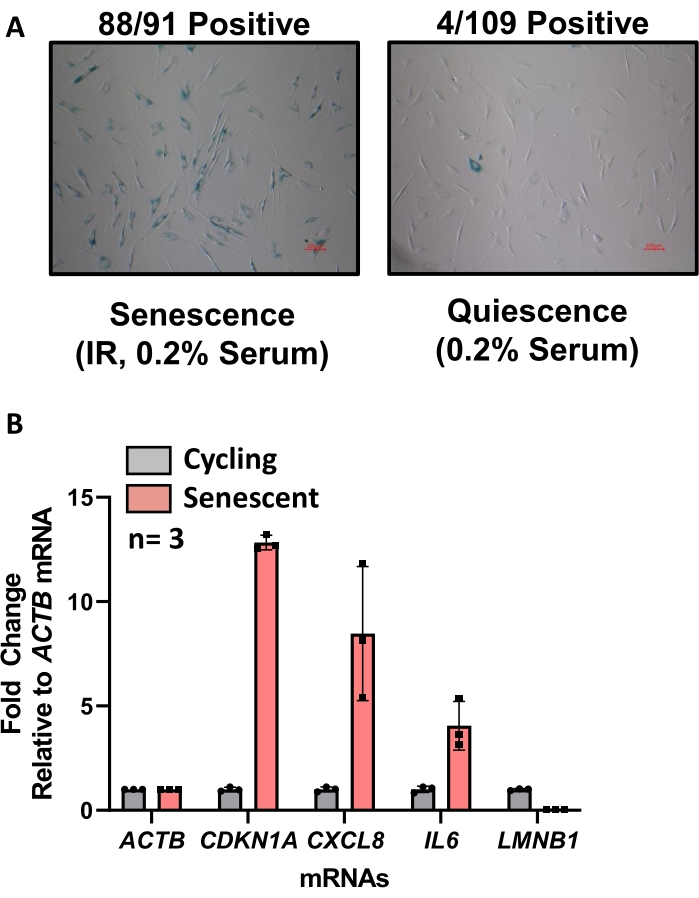
Figure 2: Validation of senescent phenotypes using SA-βGal and RT-qPCR analyses. (A) Senescent and quiescent cells are re-plated at the time of harvest into a 6-well plate and stained for SA-βGal using the SA-βGal staining kit. Blue color in the cell body is positive for senescence. Images are taken in brightfield with color at 10x magnification, size marker in red. (B) RT-qPCR analysis comparing senescent cells (red) and cycling cells (gray) from an unrelated experiment. Increases in the levels of CDKN1A/p21, CXCL8, and IL6 mRNAs are indicative of senescence, as is the decrease in LMNB1 mRNA levels. Please click here to view a larger version of this figure.
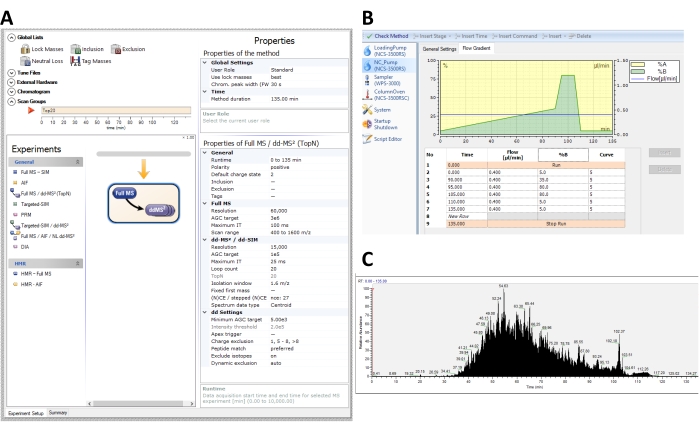
Figure 3: Representative methods for data-dependent acquisition (DDA) scans and liquid chromatography gradient of pSILAC cultures on the Q-Exactive HF orbitrap mass spectrometer. (A) Recommended instrument settings in the instrument software for data-dependent analysis of whole-cell lysates from a pSILAC experiment. (B) Example liquid chromatography flow gradient method settings. Peptides are eluted over a 90-min linear gradient ranging from 5% to 35% buffer B (0.2 % formic acid and 99.8% acetonitrile), followed by a 10 min wash with 80% buffer B, and 25 min of equilibration with 5% buffer B. (C) A representative total ion chromatogram (TIC) of a mass spectrometry acquisition of IMR-90 fibroblast peptides acquired with the specified settings. Please click here to view a larger version of this figure.
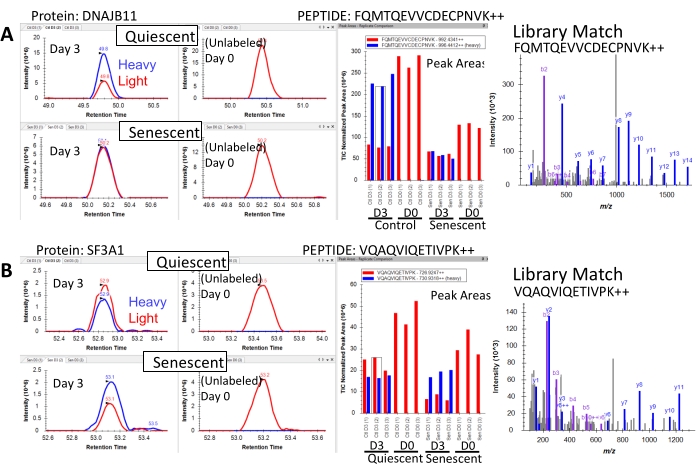
Figure 4: Representative extracted ion chromatograms of peptides with altered turnover during senescence. (A) Chromatographic peak areas of the peptide FQMTQEVVCDECPNVK++ from the protein DnaJ homolog subfamily B member 11 (DNAJB11) in senescent and non-senescent cells. Following 3 days of SILAC, senescent cells incorporate less heavy isotope into this peptide compared with quiescent (non-senescent) cells, as indicated by a reduction in the peak area of heavy isotope-containing peptide (blue) relative to the light peptide (red), indicating that this peptide has reduced turnover in senescent cells. (B) Chromatographic peak areas of the peptide VQAQVIQETIVPK++ from the protein Splicing Factor 3a Subunit 1 (SF3A1) in senescent and non-senescent cells. Following 3 days of SILAC, senescent cells incorporate a higher proportion of heavy isotope into this peptide compared with quiescent (non-senescent) cells, as indicated by a reduction in the peak area of heavy isotope-containing peptide (blue) relative to the light peptide (red), indicating that this peptide has increased turnover in senescent cells. The unlabeled (Day 0) conditions show no incorporation of heavy isotope for both peptides, as expected. Please click here to view a larger version of this figure.
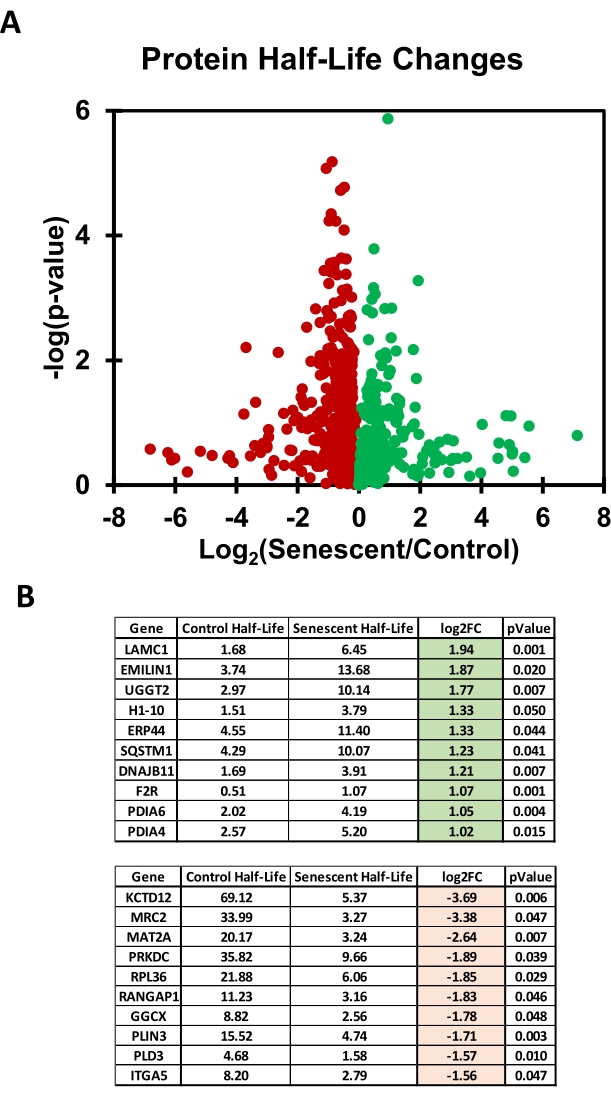
Figure 5: Comparison of protein half-lives in senescent and quiescent cells determined from pSILAC labeling. (A) Volcano plot displaying the log2 ratio of senescent / control (quiescent) for each of the 695 identified proteins; in this experiment, Light and Heavy labeling media contained no glucose and no phenol red. (B) Tables showing the top 10 proteins with the most increased or decreased half-lives in quiescent cells versus senescent cells (left and right, respectively). Please click here to view a larger version of this figure.
Table 1: Media and buffers used in this protocol. Please click here to download this Table.
Table 2: RT-qPCR primers used in this protocol. Please click here to download this Table.
Table 3: SILAC analysis workbook for calculation of protein half-lives, fold changes, and t-tests. Please click here to download this Table.
Discussion
pSILAC is a powerful technique that enables the global quantification of protein turnover rates across multiple cellular conditions. This paper details the use of pSILAC to compare global protein half-lives between senescent and quiescent cells, including instructions for the preparation of senescent and quiescent cells, SILAC labeling and harvesting, and ultimately analysis using DDA mass spectrometry. Additionally, a two-step test is described for validation of the senescence phenotype using SA-βGal and RT-qPCR analysis of a panel of mRNAs encoding senescence-associated proteins. In addition to validation of senescence with the two approaches described, a third validation of senescence can be performed following mass spectrometry analysis by looking for changes in known senescence markers between senescent and quiescent cells at the proteomic level. Senescence-associated proteins that are expected to be elevated include p16, p21, and BCL2, among others, described elsewhere44,45. In the protocol described above, ionizing radiation was used for the induction of senescence and serum starvation for quiescent cells. For the induction of senescence, there are multiple options available and there is substantial heterogeneity among them41,42,46. Currently, there is no senescent method that is considered the "most physiological", so the choice of senescent inducer is largely based on the context of the experiment. However, experiments with the goal of stating a general phenomenon about senescence are recommended to use at least two different senescent inducers. Discussing the range of senescence paradigms is beyond the scope of this paper, but some common methods to induce senescence include triggering DNA damage (IR, doxorubicin, replicative exhaustion), expressing oncogenic proteins (HRAS, BRAF), and disrupting mitochondria function2.
In addition to the choice of senescent inducer, the choice of control cells is an equally important consideration. Senescent cells are, by definition, under indefinite growth arrest, so a comparison to other growth-arrested cells are often chosen. For pSILAC, cell cycle arrested cells are generally preferable because they do not replicate and are thus easier to use for protein half-life calculations47. However, since cultured cells will often retain some dividing cells, it is important that the methods used to induce cell cycle arrest produce as homogenous a response as possible to minimize error from cells that are still proliferating. To calculate protein degradation rates for cycling cells using pSILAC requires additional calculations to compensate for the rate at which protein is diluted into daughter cells27. However, quiescent growth arrest itself is not without complications. There are two general methods for cell cycle arrest: serum deprivation and contact inhibition48. Not all cells can be made quiescent through contact inhibition, although some fibroblasts have been shown to demonstrate quiescence after several days of culturing49. This method used serum deprivation because it is more commonly used for comparisons of senescent cells, though it requires for the senescent cell to be similarly serum-deprived for accurate comparisons. Serum activates the mTOR complex, and thus serum deprivation has several downstream effects on the cell in addition to cell cycle arrest50. Notably, senescent cells have been shown to display a reduced SASP upon serum deprivation or mTOR inhibition51,52.
Another important point to consider in pSILAC is how many time points to test. This protocol collected cells at a single time point (3 days of light or heavy labeling), which substantially simplifies the resulting analysis. The choice of time point should be based on the objective of the experiment. For global analysis, 3 days is expected to capture a majority of proteins, though half-lives for short-lived proteins that completely turnover within 3 days (all light signal is lost) cannot be measured at this time point. Conversely, long-lived proteins with very little turnover in 3 days are also difficult to quantify and often appear as having extremely large half-lives (in the order of weeks) that are typically just a consequence of very little heavy signal accumulation. Due to the non-linear relationship in the ratio of heavy and light peptide signals versus the percentage of newly synthesized protein at shorter and longer time points, the quantitation of half-lives could be improved by adding additional labeling time points. For relative comparisons between two cell states, as in this protocol, an approximate half-life may be sufficient, but additional timepoints can be used to improve quantitative accuracy.
This protocol describes how to perform an untargeted DDA-based analysis of protein turnover. However, the protein turnover calculations can be applied generally to any acquisition scheme that is able to derive the relative abundance of heavy and light peptide pairs. For example, MS2-based methods such as data-independent acquisition (DIA/SWATH) can also be applied for the calculation of turnover rates successfully53. Additionally, instrumentation and software pipelines other than those described in this protocol can be used to perform DDA analysis, protein identification, and protein quantification. When using protein quantification software platforms such as Skyline to extract peptide peak areas, it is advisable to manually inspect extracted ion chromatograms in the document workspace, identify peaks that were erroneously integrated and non-quantitative peaks, and curate the document accordingly. An extensive collection of tutorials is available online for Skyline (skyline.ms).
pSILAC represents one of the most ideal methods for global quantification of protein half-lives in cultured cells due to superior multiplexing (proteome coverage) and throughput. While pSILAC does not provide direct rates of synthesis or degradation, since the change in light and heavy signal is due to a confluence of factors, pSILAC is highly useful for comparisons between conditions and different cell types. Low-throughput methods often fall into two types: 1) treatment of cells with cycloheximide to block protein synthesis and harvest at time intervals after addition to monitor decay, or 2) treatment of cells with an inhibitor of protein decay and harvest at time intervals after addition to monitor the accumulation of protein, thus inferring protein decay rates. The limitation of both methods is that such treatments will inevitably cause substantial changes to cellular physiology. In contrast, pSILAC requires no substantial intervention and theoretically has no detectable effects on cellular physiology since isotopic amino acids differ by only a single neutron from their non-isotopic counterparts. Thus, the method described here for pSILAC represents a simple protocol for global measurement of the most physiological protein half-lives in non-dividing cells.
Alterations in protein turnover have a close relationship to aging, age-related diseases, neurodegeneration, and longevity54,55. This protocol describes a method to interrogate these relationships by using stable isotope labeling of amino acids in cell culture to measure protein turnover rates in senescence cells. However, numerous analogous methods exist to perform studies in the context of aging and neurodegeneration in vivo in whole organisms such as mice. Indeed, these studies have emphasized the importance of measuring protein turnover rates in the context of age-related diseases56,57,58,59.
In this study, ribosomal proteins and proteins residing in the endoplasmic reticulum stood out as two categories of proteins with decreased and increased half-lives in senescent cells, respectively. While further analysis of steady-state levels is required for definitive conclusions, these results further suggest that senescent cells may uniquely regulate translation through decreased half-lives of ribosomal proteins. Going forward, applying stable isotope labeling approaches to study the relationship between cellular senescence and neurodegeneration in vivo in mouse models will be a promising extension of the isotope labeling approach described by this protocol.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) and the Intramural Research Program (IRP), National Institute on Aging (NIA). N.B. was supported by Longevity Impetus Grants, and the Office of Dietary Supplements (ODS) Scholars Program. Figure 1 was created with BioRender.com.
Materials
| Acetonitrile (LC-MS grade) | Grainger | AH015 | |
| Ammonium Bicarbonate | Millipore-Sigma | 9830 | |
| Antibiotic-Antimycotic (100x) | ThermoFisher | 15240062 | |
| BCA Assay Kit | ThermoFisher | 23227 | |
| Dithiothreotol (DTT) | Sigma | D9779 | |
| DMEM, high glucose, HEPES | ThermoFisher | 12430112 | |
| dNTP Mix | ThermoFisher | R0191 | |
| Fetal Bovine Serum, certified, heat inactivated | ThermoFisher | 10082147 | |
| Formic Acid | Sigma | 27001 | |
| Gammacel 40 Exactor | Best Theratronics | Cesium Irradiator for cells | |
| GlycoBlue | ThermoFisher | AM2238 | |
| Iodoacetamide (IAA) | Sigma | I1149 | Light sensitive |
| IMR-90 primary lung fibroblasts | ATCC | CCL-186 | |
| iRT Kit (indexed retention time) | Biognosys | Ki-3002-2 | Indexed Retention Time Peptide Standards |
| Isopropanol | ThermoFisher | 423835000 | |
| Mascot | Matrix Science | Mascot Daemon 2.8 | Proteomic database searching software |
| Maxima Reverse Transcritase (200 U/µL) | ThermoFisher | EP0742 | |
| MEM Non-Essential Amino Acids Solution (100x) | ThermoFisher | 11140050 | |
| Nano LC System | ThermoFisher | ULTIM3000RSLCNANO | |
| Oasis HLB Solid Phase Extraction Cartirdges | Waters | 186000383 | |
| Orbitrap Mass Spectrometer | ThermoFisher | Q Exactive HF Orbitrap | |
| Phenol/Chloroform/Isoamyl alcohol (25:24:1), 100 mM EDTA, pH 8.0 | ThermoFisher | 327110025 | |
| Phosphate Buffered Saline (PBS) | ThermoFisher | 10010023 | |
| Pierce SILAC Protein Quantitation Kit (Trypsin) -DMEM | ThermoFisher | A33972 | |
| QuantStudio 6 Real-Time PCR System | ThermoFisher | ||
| Random Hexamer Primer | ThermoFisher | SO142 | |
| Senescence β-Galactosidase Staining Kit | Cell Signaling | 9860 | |
| Skyline | University of Washington | Skyline-Daily v21.2.1.424 | Free and open source qantiative proteomic software. Available on www.skyline.ms |
| Sonicator waterbath | Branson | CPX-952-516R | |
| TRIzol Reagent | ThermoFisher | 15596018 | Referred to as phenol in text; hazardous |
| TRYPle Express | ThermoFisher | 12605010 | |
| Trypsin (sequencing grade) | Promega | V5113 | |
| TURBO Dnase (2U/ uL) | ThermoFisher | AM2238 | |
| Urea | ThermoFisher | 29700 | |
| Water (LC-MS grade) | Grainger | AH365 |
References
- Hayflick, L. The cell biology of aging. Journal of Investigative Dermatology. 73 (1), 8-14 (1979).
- Gorgoulis, V., et al. Cellular senescence: Defining a path forward. Cell. 179 (4), 813-827 (2019).
- Borghesan, M., Hoogaars, W. M. H., Varela-Eirin, M., Talma, N., Demaria, M. A senescence-centric view of aging: Implications for longevity and disease. Trends in Cell Biology. 30 (10), 777-791 (2020).
- Martinez-Cue, C., Rueda, N. Cellular senescence in neurodegenerative diseases. Frontiers in Cellular Neuroscience. 14, 16 (2020).
- Wissler Gerdes, E. O., et al. Cellular senescence in aging and age-related diseases: Implications for neurodegenerative diseases. International Review of Neurobiology. 155, 203-234 (2020).
- Baker, D. J., Petersen, R. C. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. Journal of Clinical Investigation. 128 (4), 1208-1216 (2018).
- Musi, N., et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 17 (6), 12840 (2018).
- Ogrodnik, M., et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metabolism. 29 (5), 1061-1077 (2019).
- Chow, H. M., et al. Age-related hyperinsulinemia leads to insulin resistance in neurons and cell-cycle-induced senescence. Nature Neuroscience. 22 (11), 1806-1819 (2019).
- Dehkordi, S. K., et al. Profiling senescent cells in human brains reveals neurons with CDKN2D/p19 and tau neuropathology. Nature Aging. 1, 1107-1116 (2021).
- Chinta, S. J., et al. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Reports. 22 (4), 930-940 (2018).
- Bussian, T. J., et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 562 (7728), 578-582 (2018).
- Zhang, P., et al. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nature Neuroscience. 22 (5), 719-728 (2019).
- Selkoe, D. J., Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Molecular Medicine. 8 (6), 595-608 (2016).
- Wei, Z., et al. Amyloid beta protein aggravates neuronal senescence and cognitive deficits in 5xfad mouse model of Alzheimer’s disease. Chinese Medical Journal (England). 129 (15), 1835-1844 (2016).
- He, N., et al. Amyloid-beta(1-42) oligomer accelerates senescence in adult hippocampal neural stem/progenitor cells via formylpeptide receptor 2. Cell Death & Disease. 4 (11), 924 (2013).
- van Dijk, K. D., et al. Changes in endolysosomal enzyme activities in cerebrospinal fluid of patients with Parkinson’s disease. Movement Disorders: Offical Journal of Movement Disorder Society. 28 (6), 747-754 (2013).
- Chinta, S. J., et al. Environmental stress, ageing and glial cell senescence: a novel mechanistic link to Parkinson’s disease. Journal of Internal Medicine. 273 (5), 429-436 (2013).
- Coppe, J. P., et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biology. 6 (12), 2853-2868 (2008).
- Coppe, J. P., Desprez, P. Y., Krtolica, A., Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual Review of Patholology. 5, 99-118 (2010).
- Acosta, J. C., et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology. 15 (8), 978-990 (2013).
- Payea, M. J., Anerillas, C., Tharakan, R., Gorospe, M. Translational control during cellular senescence. Molecular and Cellular Biology. 41 (2), 00512 (2021).
- Nishimura, K., et al. Perturbation of ribosome biogenesis drives cells into senescence through 5S RNP-mediated p53 activation. Cell Reports. 10 (8), 1310-1323 (2015).
- Lessard, F., et al. Senescence-associated ribosome biogenesis defects contributes to cell cycle arrest through the Rb pathway. Nature Cell Biology. 20 (7), 789-799 (2018).
- Xu, M., et al. Senolytics improve physical function and increase lifespan in old age. Nature Medicine. 24 (8), 1246-1256 (2018).
- Wiley, C. D., et al. SILAC Analysis reveals increased secretion of hemostasis-related factors by senescent cells. Cell Reports. 28 (13), 3329-3337 (2019).
- Schwanhausser, B., et al. Global quantification of mammalian gene expression control. Nature. 473 (7347), 337-342 (2011).
- Doherty, M. K., Hammond, D. E., Clague, M. J., Gaskell, S. J., Beynon, R. J. Turnover of the human proteome: determination of protein intracellular stability by dynamic SILAC. Journal of Proteome Research. 8 (1), 104-112 (2009).
- Riba, A., et al. Protein synthesis rates and ribosome occupancies reveal determinants of translation elongation rates. Proceedings of the National Academy of Sciences of the United States of America. 116 (30), 15023-15032 (2019).
- Mathieson, T., et al. Systematic analysis of protein turnover in primary cells. Nature Communications. 9 (1), 689 (2018).
- Liu, T. Y., et al. Time-resolved proteomics extends ribosome profiling-based measurements of protein synthesis dynamics. Cell Systems. 4 (6), 636-644 (2017).
- Welle, K. A., et al. Time-resolved analysis of proteome dynamics by tandem mass tags and stable isotope labeling in cell culture (TMT-SILAC) hyperplexing. Molecular & Cellular Proteomics: MCP. 15 (12), 3551-3563 (2016).
- Neri, F., Basisty, N., Desprez, P. Y., Campisi, J., Schilling, B. Quantitative proteomic analysis of the senescence-associated secretory phenotype by data-independent acquisition. Current Protocols. 1 (2), 32 (2021).
- Hernandez-Segura, A., Brandenburg, S., Demaria, M. Induction and validation of cellular senescence in primary human cells. Journal of Visualized Experiments: JoVE. (136), e57782 (2018).
- Noren Hooten, N., Evans, M. K. Techniques to Induce and quantify cellular senescence. Journal of Visualized Experiments: JoVE. (123), e55533 (2017).
- Debacq-Chainiaux, F., Erusalimsky, J. D., Campisi, J., Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nature Protocols. 4 (12), 1798-1806 (2009).
- Livak, K. J., Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25 (4), 402-408 (2001).
- Hernandez-Segura, A., Rubingh, R., Demaria, M. Identification of stable senescence-associated reference genes. Aging Cell. 18 (2), 12911 (2019).
- MacLean, B., et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 26 (7), 966-968 (2010).
- Pino, L. K., et al. The Skyline ecosystem: Informatics for quantitative mass spectrometry proteomics. Mass Spectrometry Reviews. 39 (3), 229-244 (2020).
- Casella, G., et al. Transcriptome signature of cellular senescence. Nucleic Acids Research. 47 (14), 7294-7305 (2019).
- Basisty, N., et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biology. 18 (1), 3000599 (2020).
- Perkins, D. N., Pappin, D. J., Creasy, D. M., Cottrell, J. S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 20 (18), 3551-3567 (1999).
- Hernandez-Segura, A., Nehme, J., Demaria, M. Hallmarks of cellular senescence. Trends in Cell Biology. 28 (6), 436-453 (2018).
- Kohli, J., et al. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nature Protocols. 16 (5), 2471-2498 (2021).
- Hernandez-Segura, A., et al. Unmasking transcriptional heterogeneity in senescent cells. Current Biology: CB. 27 (17), 2652-2660 (2017).
- Swovick, K., et al. Interspecies differences in proteome turnover kinetics are correlated with life spans and energetic demands. Molecular & Cellular Proteomics: MCP. 20, 100041 (2021).
- Marescal, O., Cheeseman, I. M. Cellular mechanisms and regulation of quiescence. Developmental Cell. 55 (3), 259-271 (2020).
- Lacorazza, H. D. . Cellular Quiescence. , (2018).
- Saxton, R. A., Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell. 168 (6), 960-976 (2017).
- Herranz, N., et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nature Cell Biology. 17 (9), 1205-1217 (2015).
- Laberge, R. M., et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nature Cell Biology. 17 (8), 1049-1061 (2015).
- Pino, L. K., Baeza, J., Lauman, R., Schilling, B., Garcia, B. A. Improved SILAC quantification with data-independent acquisition to investigate Bortezomib-induced protein degradation. Journal of Proteome Research. 20 (4), 1918-1927 (2021).
- Basisty, N., Meyer, J. G., Schilling, B. Protein turnover in aging and longevity. Proteomics. 18 (5-6), 1700108 (2018).
- Basisty, N., Holtz, A., Schilling, B. Accumulation of "Old Proteins" and the critical need for MS-based protein turnover measurements in aging and longevity. Proteomics. 20 (5-6), 1800403 (2020).
- Basisty, N., et al. Mitochondrial-targeted catalase is good for the old mouse proteome, but not for the young: ‘reverse’ antagonistic pleiotropy. Aging Cell. 15 (4), 634-645 (2016).
- Dai, D. F., et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 13 (3), 529-539 (2014).
- Karunadharma, P. P., et al. Subacute calorie restriction and rapamycin discordantly alter mouse liver proteome homeostasis and reverse aging effects. Aging Cell. 14 (4), 547-557 (2015).
- Basisty, N. B., et al. Stable isotope labeling reveals novel insights into ubiquitin-mediated protein aggregation with age, calorie restriction, and rapamycin treatment. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 73 (5), 561-570 (2018).

