Surgical Technique of the 3-Dimensional-printed Personalized Hip Implant for the Treatment of Canine Hip Dysplasia
Summary
This work describes a novel surgical technique for extracapsular implantation of a personalized, 3-dimensional-printed, joint-preserving implant. This novel treatment aims to restore hip stability in young adult dogs suffering from hip dysplasia with laxity by uniquely reproducing the anatomical shape of the acetabular rim of the hip joint.
Abstract
Hip dysplasia causes major disability in dogs. Treatment options are limited to palliative treatment (e.g., pain relief, physical exercise, lifestyle changes, and weight control) or invasive surgeries such as pelvic osteotomies and total hip arthroplasty. Hence, a strong unmet need exists for an effective and dog-friendly solution that enhances the quality of life of man's best friend. We fill this treatment gap by offering a minimally traumatic and extraarticular, dog-specific, 3-dimensional-printed, hip implant (3DHIP) that restores hip joint stability. The surgical treatment using a 3DHIP implant is less invasive than osteotomies and can be performed bilaterally in one surgical session. The 3DHIP implant extends the dorsal acetabular rim of the dysplastic hip joint thereby increasing coverage of the femoral head and inhibiting joint subluxation with fast recovery. Sufficient access to the dorsal acetabular rim and ventral border of the iliac body together with optimal fitting and fixation of the implant are key steps for a successful 3DHIP implantation and imply the need for a specific approach. The present article aims to showcase this innovative surgical technique with tips and tricks as a surgical manual for implantation of the 3DHIP implant in dogs affected by hip dysplasia.
Introduction
Hip dysplasia (HD) in dogs manifests due to a bad fit between the hip socket (acetabulum) and the femoral head resulting in subluxation of the hip joint. It affects mainly young medium- to large-breed dogs, resulting in joint cartilage deterioration, and ultimately, severe osteoarthritis (OA) leading to chronic pain and low quality of life1,2. The overall prevalence of hip dysplasia in dogs is 15.56%, which varies widely based on breed and classification systems3,4.
Apart from lifestyle changes, hip dysplastic dog patients are treated with antiinflammatory and analgesic drugs to control pain and maintain mobility4. In case of hip laxity in young adult dogs, the only surgical resort is double (DPO) or triple pelvic osteotomy (TPO), a procedure involving two or three full cuts of the pelvic bones to expand the coverage of the femoral head. However, complications after osteotomies are common and the progression of OA is still observed5,6,7,8,9. Once severe OA and chronic pain have developed, only high-impact complex surgery like total hip replacement (THR) or salvage femoral head and neck ostectomy (FHO) remain10. However, FHO presents less favorable outcomes in large breed dogs and necessitates prolonged physical therapy for the restoration of limb function11. Furthermore, THR is technically challenging and inherently associated with severe complications12,13,14. Therefore, effective hip dysplasia therapy necessitating only low-impact surgery and with a lower complication risk is required before this end stage is achieved.
The 3-dimensional (3D)-printed personalized hip implant (3DHIP) is a first-of-a-kind treatment for canine hip dysplasia developed with the intention to offer a minimal traumatic dog-specific implant that restores hip joint stability. The technique involves a titanium implant to treat mainly young adult (6 months to 2 years old) dog patients with a dysfunctional hip joint showing hip laxity grade B (borderline) to D (moderate) according to the Fédération Cynologique Internationale (FCI)15. After computed tomography (CT) imaging of the dysplastic joint, an implant is designed following the specific anatomy of the hip joint in a personalized manner to extend the dorsal acetabular rim, thereby preventing hip joint subluxation and restoring hip joint stability.
A previous canine cadaver study revealed that the implant enhanced femoral head coverage and demonstrated failure under an impact force of 1,330 ± 320 Newtons16. Subsequently, a pilot study with experimental dogs demonstrated enhanced femoral head coverage, reduced hip laxity, and increased weight bearing by force plate analysis. Furthermore, examination of the intervened hips at 6 months post-implantation revealed normal volume and a smooth surface of both the femoral head and acetabulum cartilage, accompanied by joint capsule hypertrophy based on gross and histological assessments17. Upon confirmation of the efficacy and safety of the implant and treatment concept, a clinical investigation was carried out on client-owned dogs suffering from hip dysplasia. The short-term study revealed that the benefits of the 3D-printed acetabular rim extension implant are a personalized, good fit of the implant to the acetabulum restoring hip joint stability, decreased pain-related activities, and a low-impact surgical procedure18. Application of the implant requires access to the ventrocaudal aspect of the iliac body and the craniodorsal aspect of the hip joint. In this paper, we describe our surgical planning and surgical procedure with a modified craniodorsal approach to the hip joint as a manual for implantation of the 3DHIP in dogs affected by hip dysplasia.
Protocol
This study was considered a non-experimental clinical veterinary practice as mentioned in Article 1 – 5(b) of Directive 2010/63/EU and was approved by the Veterinary Clinical Studies Committees (VCSC), Utrecht University, Utrecht, The Netherlands. This study involved the treatment of client-owned dogs, with all dogs continuing under the care of their respective owners. All dog owners were provided with an information letter detailing the study protocol, all potential complications (e.g., infection, implant failure, neurological deficits, and others), and alternative treatments like pelvic osteotomy. Furthermore, in this form, privacy aspects and inherent data management were explained. All clients signed an informed consent form. The entire protocol of this study is divided into the following major steps: patient selection, 3DHIP implant design and production, preoperative management and anesthesia, surgical procedure, and post-operative management.
1. Patient selection
- Identify client-owned dogs > 6 months old with clinical signs related to HD that have a positive Ortolani subluxation sign (Figure 1) and radiographic evidence of HD with FCI grade B to D (Figure 2).
NOTE: The Ortolani subluxation test is a diagnostic maneuver used in veterinary medicine to assess hip joint stability in dogs. During the Ortolani test in dogs, the examiner positions the animal on its back and flexes the hips to 90° while stabilizing the pelvis. Each hind limb is gently abducted, aiming to displace the femoral head from the acetabulum to assess hip joint stability. A positive Ortolani sign, indicated by a characteristic "clunk" or movement, suggests hip dysplasia and the potential for hip joint instability. - Exclude the dogs with an open acetabular growth plate, luxoid hips, or prior hip surgery.
- Perform a CT scan of the hips to exclude dogs with moderate to severe osteoarthritic changes of the hips and for implant design.
NOTE: Dogs that have femoral neck and/or cranial and caudal acetabular rim osteophytes > 2 mm are excluded (Figure 3).
2. 3DHIP implant design and production
- Design the 3DHIP implant (patent number EP3463198B119) from preoperative CT DICOM images of the complete pelvis as described by Willemsen et al.16 and Kwananocha et al.18 at the laboratory (refer to the Table of Materials).
- Segment the pelvic bone and femora of each candidate dog from the preoperative CT and create a 3D model using specialized software (refer to the Table of Materials and Figure 4A).
- Create a local coordinate system for the pelvis, based on the inclinated posterior pelvic plane.
- Measure the native Norberg angle (NA) of each candidate dog's hips on the 3D model (Figure 4B).
- Use the specialized software (refer to the Table of Materials) to design the 3DHIP implant on the 3D model of the pelvis. Use the native NA to determine the amount of extension of the dorsal acetabular rim that is needed; the 3DHIP implant increases the NA by 25-35 degrees (Figure 4C and Figure 4D).
- Ensure that the 3DHIP implant consists of two subsections: the attachment part and the rim extension part. Design the attachment part to consist of a porous inner shell and incorporate four locking screw holes and an additional ventral ilium flange for ease and precision of positioning. Design the rim extension part to extend the dorsal acetabular rim with an internal 1.5 mm offset allowing unhindered joint capsule attachment (Figure 5A and Figure 5B).
- Print the created 3DHIP implant (Figure 5C) from medical grade titanium alloy (Ti6AI4V ELI grade 23) using selective laser melting technology with a direct metal printing machine, operated by the implant manufacturer (refer to the Table of Materials).
- Postprocess the printed 3DHIP implant, including stress release (vacuum annealing), manual mirror polishing, and ultrasonic cleaning, operated by the implant manufacturer (refer to Table of Materials).
- Perform final cleaning at the hospital sterilization unit to eliminate any residual metal dust leftover from the manufacturing process.
- Manually wash the 3DHIP implant using povidone-iodine shampoo (refer to the Table of Materials) and sterile water.
- Clean the 3DHIP implant using the hygienic washing machine (refer to the Table of Materials) at a temperature of 94 °C for 90 min.
- Enclose the 3DHIP implant in double transparent sterilization laminates (refer to the Table of Materials) and seal each laminate separately.
- Sterilize the implant using steam autoclaving (refer to the Table of Materials) at a temperature of 134 °C for 80 min.
3. Preoperative management and anesthesia
- Perform a general examination of the client-owned dog before anesthesia and categorize the anesthesia risk using the American Society of Anesthesiologists (ASA) patient scale (scale 1-5)20.
NOTE: The American Society of Anesthesiologists (ASA) patient scale is employed to categorize the anesthesia risk of dogs and assists veterinarians in determining appropriate anesthetic protocols for each dog. It classifies dogs into different classes based on their health status, ranging from Class I for healthy dogs to Class V for those in critical condition not expected to survive surgery. Dogs categorized under ASA 1 (normal healthy dog) and ASA 2 (dogs with mild systemic disease) are considered suitable candidates for this surgical treatment. These dogs typically have no or mild underlying systemic diseases, are in good overall health, and exhibit normal physiological functions. They are considered low-risk candidates for surgery and anesthesia. The animal needs to be fasted for at least 6 h before the scheduled induction time. - Anesthetize the dog following ASA categorization on the individual patient's needs.
- Administer intravenous dexmedetomidine at a dose of 2 µg/kg and methadone hydrochloride at a dose of 0.3 mg/kg for premedication in the dog. (refer to the Table of Materials).
NOTE: The selection of premedications and the route of administration may differ depending on the anesthesiologist's preference and the dog's health condition and behavior. - Administer intravenous propofol at a dosage of 2-4 mg/kg (refer to the Table of Materials) for anesthesia induction.
- Intubate the dog and sustain anesthesia with inhaled isoflurane (refer to the Table of Materials) and oxygen.
- Administer intravenous dexmedetomidine at a dose of 2 µg/kg and methadone hydrochloride at a dose of 0.3 mg/kg for premedication in the dog. (refer to the Table of Materials).
- Continuously monitor and ensure the stability of vital signs, encompassing heart rate, respiratory rate, end-expiratory carbon dioxide levels, percutaneous arterial oxygen saturation, non-invasive arterial blood pressure, esophageal temperature, and electrocardiography.
- Perform epidural analgesia using a sterile technique.
- Position the dog in sternal recumbency and gently flex the hind limbs forward to create more space between the last lumbar vertebra and the sacrum.
- Identify the injection site located just caudal to the 7th dorsal spinal process, where a "dimple" can be palpated.
- Conduct a sterile scrub and don sterile gloves using aseptic techniques.
- Insert the spinal needle tip into the epidural space and verify its correct placement using the "hanging drop" technique. Briefly, introduce a drop of saline into the spinal needle hub. When the spinal needle tip penetrates the ligamentum flavum and enters the epidural space, the saline within the hub will migrate from the hub into the needle21.
- Inject the drugs (morphine 0.1 mg/kg diluted with levobupivacaine 1 mL/5 kg) (refer to the Table of Materials) at a slow, constant rate to ensure even distribution of the drug when correct placement is confirmed.
- Place an indwelling Foley urinary catheter (refer to the Table of Materials) in the bladder using a sterile technique and leave it in place for 12-24 h.
- Ensure a clean and sterile environment for the catheterization procedure; trim the hair on the prepuce in male dogs and the surrounding ventral vaginal vault in female dogs.
- Cleanse the area with povidone-iodine solution (refer to the Table of Materials) and flush the prepuce/vaginal vault with 2-12 mL of diluted povidone-iodine solution (the volume varies based on the size of the dog).
- Wash hands thoroughly and wear sterile gloves to minimize the risk of contamination.
- Apply sterile lubricating jelly to the distal end of the Foley catheter and employ aseptic technique during the insertion of the Foley catheter.
- Once the catheter is correctly positioned in the bladder, inflate the balloon with sterile water according to the volume specified on the package. This secures the catheter in place and prevents accidental dislodgement.
- Connect the drainage port on the Foley catheter to the receiving port on a urine collection bag.
- Clip the entire limb circumferentially starting from the vertebral column to just distal to the hock. In case of single-stage bilateral 3DHIP implantation, clip the other limb in a similar fashion and connect the left and right sides on the lumbosacral dorsum.
- Wrap the distal part of the limb with a non-sterile cohesive bandage. Select an appropriate width and length of non-sterile cohesive bandage, starting slightly below the surgical site and wrapping it spirally down the limb, covering the paw and nails.
- Administer cefazolin for injection at a dose of 20 mg/kg (refer to the Table of Materials) intravenously 30 min before skin incision and repeat every 90 min until the end of surgery.
- Position the dog on a standard operating table in lateral recumbency and put the affected limb into a hanging position. Secure the patient in this position using a vacuum bean bag positioner
- Conduct the final sterile surgical scrub on the limb in preparation for the surgery. Scrub the skin 2x with 4% chlorhexidine gluconate, and finish with two applications of 70% (v/v) ethanol spray (refer to the Table of Materials).
- Position four surgical waterproof drapes around the surgical site. Direct an assistant to release the distal limb from its suspended position, while the surgeon secures and covers the distal limb with a sterile waterproof sock. Add a layer of sterile cohesive wrap for extra protection.
- Cover the exposed skin of the entire surgical area with an iodine-impregnated drape (refer to the Table of Materials) and then secure with towel clamps.
4. Surgical procedure
- Identify the tip of the greater trochanter, the cranial border of the proximal femur, and the iliac wing through palpation to establish orientation.
- Incise the skin sharp with a surgical knife from the cranial dorsal iliac spine starting 6-10 cm cranial to the greater trochanter. Then, slightly turn ventrally along the cranial border of the proximal femur. Stop the incision 2-5 cm distal to the greater trochanter. The incision length is approximately 8-15 cm (depending on the size of the dog; Figure 6A).
NOTE: This surgical approach was modified from the craniodorsal approach of the hip joint previously reported by Johnson22. - Make an incision through the subcutaneous fat down to the fascia to establish an anatomic dissection following the anatomic planes (Figure 6B).
- Sharply separate and incise the superficial leaf of the fascia latae muscle along the cranial border of the biceps femoris muscle. Retract the biceps femoris muscle caudally.
- Identify the fatty triangle, which is bordered by the tensor fascia latea muscle, the gluteal muscle, and the biceps femoris muscle. Separate the fatty triangle with a blunt-tipped dissecting scissor and index finger, which will provide access to the deeper layers.
- Incise the intermuscular septum between the superficial gluteal muscle, the middle gluteal muscle, and the tensor fascia latae muscle with a surgical knife (Figure 7A and Figure 7E).
- Use a hand-held retractor to separate and retract the superficial and middle gluteal muscles dorsally, which will expose the insertion of the deep gluteal muscle.
- Undermine the deep gluteal muscle close to the greater trochanter using blunt-tipped dissecting scissors.
- Preplace a stay suture on the deep gluteal tendon approximately 1-1.5 cm proximal from its insertion on the greater trochanter.
- Perform a complete deep gluteal tenotomy close to the bone (approximately 0.5-1 cm away from its insertion) using a surgical knife (Figure 7B and Figure 7F).
- Do a blunt dissection using blunt-tipped dissection scissors to free the deep gluteal muscle from the underlying joint capsule, following which it can be subperiosteally elevated from the ilium using a periosteal elevator and index finger.
- Use bipolar electrocautery for hemostasis of small vessels between the deep gluteal muscle and the joint capsule. Then, retract the deep gluteal muscle dorsally by replacing the Amry Navy retractors.
- Partially free the iliacus muscle from the caudoventral border of the iliac shaft using a periosteal elevator and identify the insertion of the rectus femoris muscle (Figure 7C and Figure 7G).
- Use a periosteal elevator to remove all remaining soft tissue from the exposed iliac shaft to prepare for accurate positioning of the 3DHIP implant and scratch the periosteum to stimulate bone ingrowth for secondary fixation of the implant.
- Identify the articularis coxae muscle caudal to the rectus femoris muscle overlying the joint capsule.
NOTE: The articularis coxae muscle can be freed from its insertion if it interferes with implant positioning. - Fit the 3DHIP implant into its designated position with the implant's flange of the attachment part hooking under the ventral border of the exposed caudoventral iliac shaft just cranial to the bony prominence that marks the insertion of the rectus femoris muscle (Figure 7D and Figure 7H).
- Check that the rim extension part of the implant overlays the craniodorsal part of the hip joint capsule without interfering with the attachment of the joint capsule on the acetabular rim and that no deep gluteal muscle is captured under the extension part.
NOTE: Adequate exposure for implant positioning and screw insertion can be achieved by continuous abduction, external rotation, and flexion of the hip joint by an assistant to release the tension on the gluteal muscle complex facilitating the surgical exposure. - Check the implant for ideal positioning by visualizing and probing with the suction canula for perfect bone stock in all four exposed screw holes and by probing for the absence of space between the ilial flange and the caudoventral iliac shaft.
- Temporarily fix the implant in the desired position with one titanium self-tapping locking screw (2.4 mm, 2.7 mm, or 3.5 mm) (refer to the Table of Materials), which is not fully tightened to allow minimal rotational adjustments to the implant when placing the second screw.
NOTE: The sequence of placement of the four screws can be adapted according to their convenient accessibility (Figure 5C). - Conduct intraoperative fluoroscopy (refer to the Table of Materials) in lateral (Figure 8A) and latero-oblique (Figure 8B) views to comprehensively assess the implant's position and alignment. Compare the obtained fluoroscopic images with preoperative planning to ensure that the implant is positioned according to the surgical plan.
- Check that the curvature of the rim extension part of the implant is exactly congruent with the curvature of the femoral head and visible caudal and cranial acetabular rim that are not covered by the implant.
NOTE: If necessary, adjustments can be performed. The first screw is removed, the implant replaced and temporarily fixed with one screw in new bone stock, and fluoroscopy is repeated. - Insert three titanium self-tapping locking screws (2.4 mm, 2.7 mm, or 3.5 mm) in the remaining screw holes to fix the implant to the ilial shaft. After the second screw is placed, completely tighten the first screw.
- Perform a final check that all screws are hand-tightened to the locking mechanism.
- Perform flexion, extension, and abduction of the hip joint as well as the Ortolani subluxation test to exclude femoral head/neck impingement and ensure that hip laxity is reversed.
- Re-attach the cut ends of the insertional tendon of the deep gluteal muscle using a locking loop suture pattern and 1-2 mattress sutures with synthetic absorbable monofilament suture material (refer to the Table of Materials) that are intended for approximation of tissue for extended periods. Extend and internally rotate the hip joint to decrease tension on the insertional tendon of the deep gluteal muscle while suturing.
- Repair the gluteal fascia and tensor fascia latae with synthetic monofilament absorbable suture in a simple interrupted pattern.
- Close the subcutaneous tissue with synthetic monofilament absorbable suture in a simple interrupted pattern and close the skin with synthetic monofilament non-absorbable suture in a simple interrupted pattern (refer to the Table of Materials).
NOTE: If a single-stage bilateral procedure is planned, turn the dog to the other side with the untreated limb in a hanging position. Following aseptic preparation, surgical procedure steps 3.10-4.27 are performed in a similar fashion. - Perform postoperative imaging consisting of either a CT scan of the hips or hip orthogonal radiographs in latero-oblique and ventrodorsal views for final evaluation of implant positioning and screw placement (Figure 9).
5. Postoperative management
- Provide overnight inpatient care and pain management (e.g., with continuous rate infusions [CRI] of ketamine HCl 2-10 mcg∙kg-1∙min-1 or sufentanil citrate 0.1 mcg∙kg-1∙h-1 with methadone (IV, 0.2 mg/kg every 6 h, carprofen (IV, 4 mg/kg once daily), and gabapentin (PO, 10 mg/kg every 8 h) (refer to the Table of Materials).
- Allow direct postoperative short leash walking as needed to urinate and defecate on the non-slippery floor on the following day (see Supplementary Video S1).
NOTE: In case of hindlimb instability or problems rising, use a support sling or towel under the belly to support the hindlimbs. - Allow home discharge the day after surgery after removal of the Foley catheter and when voluntary urination is observed.
- Prescribe medications for pain management at home (e.g., oral medications such as carprofen 2 mg/kg BID and gabapentin 10 mg/kg every 8 h for 14 days) (refer to the Table of Materials).
NOTE: In hyperactive dogs, trazodone hydrochloride 2-5 mg/kg BID (refer to the Table of Materials), oral can be administered. This can be continued for 1-2 weeks postoperatively to ensure safe rehabilitation without high impact activities. - Prohibit high-impact activities (e.g., jumping, running, stair climbing, running with other pets, or 'rough housing') for 6 weeks postoperatively. At home, walk dogs slowly and encourage them to use the operated hindlimb(s) according to a weekly exercise scheme. Within 6 weeks postoperatively, allow the dog patient to walk with leash restraint 4-6 times daily, initially for 5-10 min each in the first 2 weeks, and then extend the duration by 5 min every 2 weeks.
- Advise professional physiotherapy and/or hydrotherapy from the second week after surgery when skin wound healing is complete.
Representative Results
Short-term results of acetabular rim extension have previously been published, arising from an ongoing observational study at the Utrecht University Department of Clinical Sciences18. From December 2019 to March 2022, a total of 61 hips from 34 dogs were included in the study. The cohort consisted of 24 males and 10 females, with a median age of 12 months (ranging from 7 to 38 months) and a median body weight of 27.3 kg (ranging from 12 to 86 kg). Seven dogs underwent surgery on a unilateral hip, while twenty dogs underwent bilateral hip surgery in a single session. Additionally, seven dogs received surgery on both hips, conducted in two separate sessions.
The previous study found a significant increase in the Norberg Angle (NA), linear percentage of femoral head coverage (LFO), and percentage of femoral head coverage (PC) immediately after implantation (Table 1). Moreover, the postoperative Ortolani subluxation sign was negative in 96.7% of operated limbs indicating that the acetabular rim extension implant restored hip congruency and diminished laxity of dysplastic hips18. Particularly, the ability to increase coverage of the femoral head without performing any re-directional osteotomy allowed physiological pelvic geometry retainment. The minimally invasive technique resulted in low incidences of complications (4.9%) in the short-term, encouraged early mobilization, and decreased pain related to activity (Table 1).
Furthermore, this technique allowed single-stage bilateral 3DHIP implant placement. Treated limb(s) were weightbearing without pelvic support within 12 to 24 h after surgery. During the 12-month monitoring period, 3 dogs required revision surgery due to either implant failure (2 dogs) or a significant advancement of osteoarthritis (1 dog). Using the presented surgical approach simultaneously with the suggested hip joint movements (abduction, flexion, and external rotation), better exposure of the ventrocaudal aspect of the iliac shaft and craniodorsal aspect of the hip joint was obtained, facilitating 3DHIP implant positioning. In addition, intra-operative fluoroscopy increased the accuracy of implant positioning.
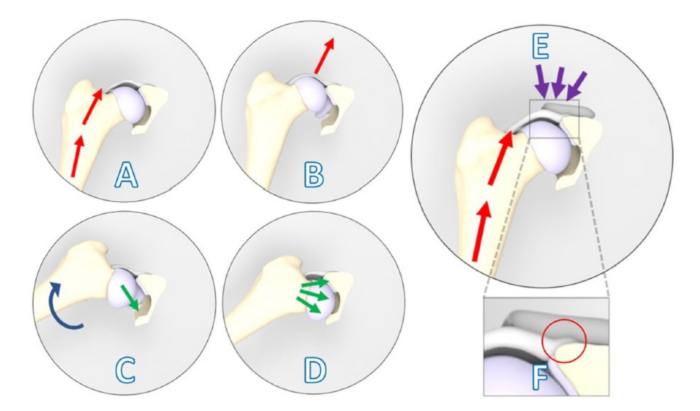
Figure 1: Schematic illustrations showing a positive Ortolani subluxation sign counteracted by the 3DHIP implant. (A–D) Positive Ortolani subluxation sign. (A) The dog's limb is positioned in neutral flexion and adduction, and a force (red arrows) is exerted towards the dorsum of the dog along the femoral axis that causes (B) dorsal subluxation of the dysplastic hip joint. (C) Gradual limb abduction (blue arrow) is performed while maintaining pressure on the femur. (D) Dependent on the acetabular rim deficiency, the subluxated femoral head falls back into the socket (green arrows). (E) The 3DHIP implant is introduced to enhance the stability of the dysplastic hip joint by reinforcing the hip capsule and labrum, which serve as weight-bearing and stabilizing surfaces (purple arrows). (F) Upon magnification of the rectangular area, the internal 1.5 mm offset of the implant is visible in the red circle, which ensures the capsule attachment remains unaffected. This figure was modified from Willemsen et al.17. Abbreviation: 3DHIP = 3-dimensional-printed, hip implant. Please click here to view a larger version of this figure.
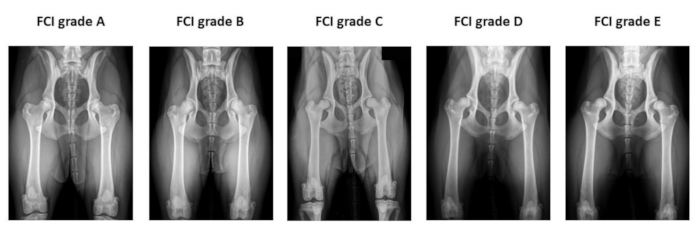
Figure 2: Example of the preoperative hip radiographs used for Fédération Cynologique Internationale hip dysplasia classification. Radiographs are taken in the ventro-dorsal hip extended position. From left to right, FCI classifies hip dysplasia into five different categories: A (normal), B (borderline), C (mild hip dysplasia), D (moderate hip dysplasia), and E (severe hip dysplasia). Abbreviation: FCI = Fédération Cynologique Internationale. Please click here to view a larger version of this figure.
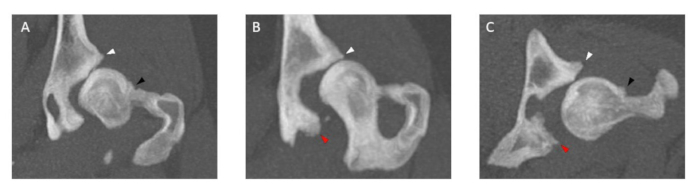
Figure 3: Images of hip joint CT examinations illustrating osteophytes of varying sizes. The thickness of all featuring osteophytes is measured in both (A,B) coronal planes and (C) transverse planes at the cranial (white arrowhead) and caudal (red arrowhead) acetabular rim and femoral neck (black arrowhead). Dogs that have femoral neck and/or cranial and caudal acetabular rim osteophytes > 2 mm are excluded. CT examination slice thickness is 5 mm. Please click here to view a larger version of this figure.
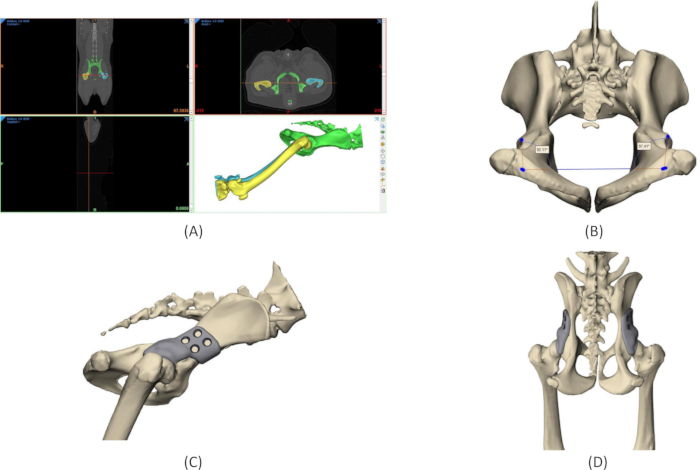
Figure 4: Design process of 3DHIP implant. (A) Segmentation of the region of interest from CT DICOM data. (B) Measurements of the native Norberg angles on the 3D model of the pelvis. (C) Rendering of a 3DHIP implant on the right hip, lateral view. (D) Rendering of bilateral 3DHIP implants, dorsal-ventral view. Abbreviation: 3DHIP = 3-dimensional-printed, hip implant. Please click here to view a larger version of this figure.
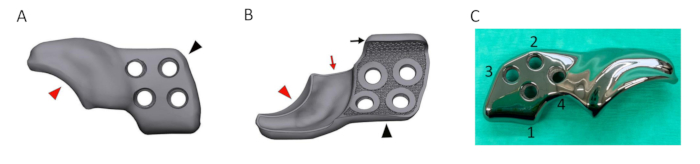
Figure 5: Rendering of a designed 3DHIP implant. (A) Rendered image of the lateral/outer side of the 3DHIP implant. (B) Rendered image of the inner implant surface showing the porous surface allowing bone ingrowth for osseointegration. The bone attachment part (black arrowhead) of rendered implant incorporating 4 locking screw holes and the ventral ilium flange (black arrow) for assisting in correct implant positioning and stabilization. The rim extension part (red arrowhead) of the rendered implant with the internal 1.5 mm offset (red arrow) allowing unhindered joint capsule attachment. (C) Photograph of a titanium 3DHIP implant showcasing 4 screw holes arranged in the sequence for screw insertion. Abbreviation: 3DHIP = 3-dimensional-printed, hip implant. Please click here to view a larger version of this figure.
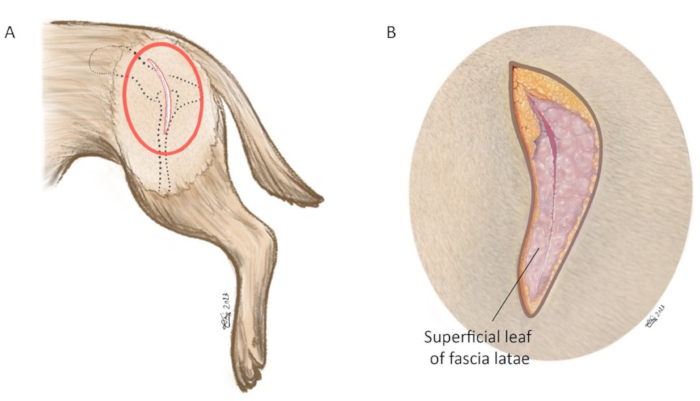
Figure 6: Schematic illustration of skin incision. (A) Red oval marks the area in which the skin incision is made. (B) Magnification of red circle in (A). The skin incision is made using a #10-blade centered on the tip of the greater trochanter aiming at the cranial dorsal iliac spine. The incision length is approximately 8-15 cm. In the magnified image, the superficial leaf of fascia latae is incised along the cranial muscle border of the biceps femoris muscle. Orientation: left is cranial, top is dorsal. Please click here to view a larger version of this figure.
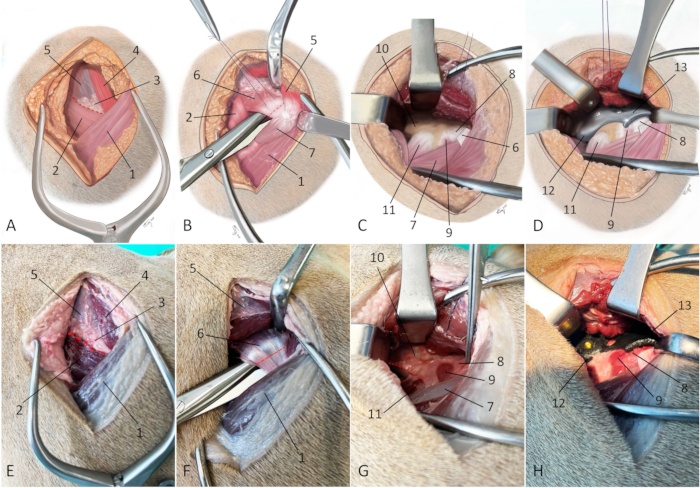
Figure 7: Schematic illustrations and photographs of an embalmed cadaver depicting the surgical approach for 3DHIP implantation. (A–D) Schematic illustrations and (E–H) photographs of an embalmed cadaver depict the surgical approach for 3DHIP implantation. (A and E) The red dotted line marks the line of the incision through the intermuscular septum between the superficial gluteal muscle, middle gluteal muscle, and the tensor fascia latae muscle. (B and F) Red dotted line marks the tenotomy site. The superficial and middle gluteal muscles are retracted dorsally to expose the deep gluteal muscle. Dissecting scissors are used to undermine the deep gluteal muscle near its insertion on the greater trochanter. A tenotomy is performed close (at 0.5-1 cm) to its insertion on the bone. (C and G) Adequate exposure for 3DHIP implant placement requires freeing the deep gluteal muscle from the joint capsule and lateral surface of the iliac body and partially freeing the iliacus muscle and rectus femoris muscles from the caudoventral border of the ilial shaft (red dotted line). (D and H) The 3DHIP implant is placed outside the capsule of the hip joint. For accuracy and ease of positioning, the ilium flange of the attachment part of the implant is placed under the ventral border of the exposed caudoventral iliac shaft. Orientation: left is cranial, top is dorsal. 1) biceps femoris muscle, 2) tensor fascia latae muscle, 3) fatty triangle, 4) superficial gluteal muscle, 5) middle gluteal muscle, 6) deep gluteal muscle/tendon, 7) vastus lateralis muscle, 8) hip joint capsule, 9) articularis coxae muscle, 10) caudal part of iliac body, 11) rectus femoris muscle, 12) ilium flange of the implant, and 13) rim extension part of the implant. Please click here to view a larger version of this figure.
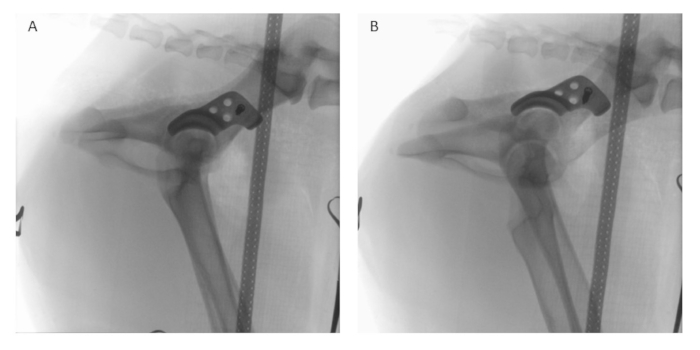
Figure 8: Intraoperative fluoroscopy. After implant positioning and temporary fixation with one locking screw, intraoperative fluoroscopy is performed in (A) lateral and (B) latero-oblique views using a digital image intensifier to assess and compare the positioning of the implant to the preoperative planning. Please click here to view a larger version of this figure.
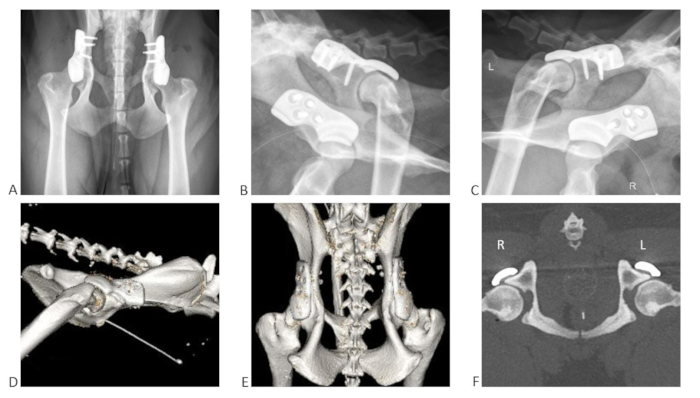
Figure 9: Examples of postoperative radiographs in three planes and postoperative CT scan after single-stage bilateral 3DHIP implant surgery in one dog. (A) Radiograph ventrodorsal view; (B) radiograph right latero-oblique view; (C) radiograph left latero-oblique view. 3D reconstruction from post-operative CT in lateral view showing the (D) right hip and (E) dorso-ventral view. (F) Postoperative CT of both hips in the transverse plane with a slice thickness of 5 mm. The 3DHIP implants were fixed with four locking screws on each side. Please click here to view a larger version of this figure.
| Outcome measurements | Preoperatively | Immediated postoperatively | 1.5 months | 3 months | p-value |
| NA (◦) | 87 ± 13a | 134 ± 19b | – | 131 ± 20b | <0.001* |
| LFO (%) | 22 ± 15a | 81 ± 16b | – | 76 ± 19b | <0.001* |
| PC (%) | 33 ± 17a | 79 ± 21b | – | 77 ± 20b | 0.002* |
| HCPI (%) | 31.44 ± 11.9a | – | 20.39 ± 10.09b | 17.69 ± 10.8b | <0.001** |
Table 1: Short-term results (Mean ± SD) of radiographic measurements using coronal CT and pain-related owner questionnaire using Helsinki Chronic Pain Index in dogs with hip dysplasia that underwent 3DHIP implantation. This table was modified from Kwananocha et al.18. HCPI (%) = 100% × total index score/maximum possible index score of the answered questions. a,bp-value < 0.05 from Bonferroni, p-value* from repeated measure analysis, p-value** from generalized linear mixed model. Abbreviations: NA = Norberg angle; LFO = linear percentage of femoral head overlap; PC = percentage of femoral head coverage; SD = standard deviation; HCPI = Helsinki Chronic Pain Index.
Supplementary Video S1: Direct postoperative weight-bearing allowed with only short leash walks on a slip-resistant floor from the day after surgery. Please click here to download this video.
Discussion
Acetabular rim extension using the 3DHIP implant provides advantages over conventional surgical therapies for canine hip dysplasia and has shown promising results to increase coverage of the dysplastic hip joint and reverse hip laxity in short-term follow-up17,18. This publication aimed to showcase the surgical technique with tips and tricks as a surgical manual for implantation of the 3DHIP implant in dogs affected by hip dysplasia.
Selection of candidates for the 3DHIP implant placement-young dogs between 6 and 24 months of age with clinical hip dysplasia marked by hip laxity (FCI grade B-D) with a positive Ortolani subluxation test are adequate candidates. The triradiate acetabular growth plate has to be closed and preferably, no osteoarthritis is present on CT imaging although minor osteophytes up to 2 mm are accepted. Dogs with luxoid hips with near-complete luxation of the femoral head are not accepted for 3DHIP implant placement because of the rapid progression of osteoarthritis, the inability of the femoral head to move into the acetabulum, and expected early conversion to total hip replacement.
There are some critical steps within the surgical technique.
Implant design
Given the individualized design of the 3DHIP implants, a preoperative assessment of the dysplastic hip joint using a CT scan is absolutely mandatory. In addition to the determination of the correct implant size and position of the ventral ilial flange, particularly the amount of acetabular rim extension needed to provide sufficient coverage of the femoral head can be determined.
Surgical approach
A critical step during surgery is sufficient exposure of the dorsal acetabular rim and ventral border of the caudal iliac body for implant placement. The surgical approach to the iliac body and craniodorsal aspects of the hip joint in 3DHIP implantation differs from conventional approaches22. In the presented technique, a trochanteric osteotomy was omitted and a deep gluteal tenotomy was performed while the superficial and middle gluteal muscles were preserved. Hereby, the risk of complications associated with trochanteric osteotomies23,24,25 such as delayed or non-union were avoided and the recovery process was expedited. Additionally, this modified craniodorsal approach can be employed across a variety of ages, breeds, and sizes of dogs without any necessary modifications. Notably, no complications were reported in association with the presented surgical approach.
Correct implant placement
Even though the custom-made 3D-printed hip implant is designed to perfectly fit the unique acetabular anatomy of each dog, imperfect implant placement with 4-5 mm craniocaudal deviation to planning target position was still observed in the first cohort of dogs possibly related to the learning curve with the technique18. The ventral ilial flange of the bone attachment part of the 3DHIP implant allows for more accurate positioning, especially in the dorsoventral direction. However, due to the extracapsular location of the implant, it is still difficult to achieve perfect implant positioning; the inner edge of the acetabulum is obscured by the synovial membrane. Further, osteophyte formation during the lead time of implant production may influence proper implant positioning. To ensure accurate implant positioning according to the preoperative plan, verification using intraoperative fluoroscopy is currently required. It is also expected that with increased experience, the precision of implant positioning will further decrease to below 1-2 mm accurate placement. In the future, guided surgery using 3D-printed surgical drill guides may obviate the need for fluoroscopy.
This technique also has some limitations. Previous short-term results suggest a broad bandwidth of different hip anatomies that can be treated using 3DHIP implants. While the long-term study findings are not yet available, it is advised to consider 3DHIP implantation for dogs that do not exhibit signs of osteoarthritis (OA) or have only a mild degree of OA in their hip joints. The 3DHIP implant placement aims to effectively slow down the progression of hip joint deterioration. Dogs with luxoid hips and moderate to severe hip degeneration as determined in the pre-operative evaluation should be excluded.
Compared to 3DHIP implantation, conventional surgeries to treat canine hip dysplasia such as DPO/TPO present more challenges, especially in a single-stage bilateral procedure and/or in giant dogs due to their invasive nature involving two or three pelvic osteotomies5,6,7,26. Therefore, dogs with bilateral HD can benefit from an acetabular rim extension using a 3DHIP implant; it provides an effective and low invasive single-stage bilateral procedure. In addition, the 3DHIP implantation helps to save valuable time and can prevent the further development of OA that might occur in double-stage bilateral procedures.
To conclude, utilization of the presented 3DHIP implant to extend the dorsal acetabular rim exhibits significant promise as an alternative surgical treatment for hip dysplasia in canines. Especially, the option to offer an effective and low invasive single-stage bilateral procedure for dogs with bilateral hip dysplasia and laxity is an enormous advantage to current alternative treatments. Further monitoring of this new technique in mid- and long-term follow-up is mandatory.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The present study was primarily financially supported by the foundation Vrienden Diergeneeskunde Universiteit Utrecht; MT has received long-term funding from the Dutch Arthritis Society (LLP22); FV and JM are funded by Eurostars Project E115515 – 3DHIP. IK is a holder of a scholarship from the Faculty of Veterinary Medicine, Kasetsart University, Thailand.
Materials
| The laborotory for implant design | |||
| 3D Lab | University Medical Center Utrecht 3D, Utrecht, Netherlands | The laboratory responsible for designing the 3DHIP implant. [https://www.umcutrecht.nl/nl/3d-lab/] | |
| Software | |||
| 3-Matic software version 17 | Materialise, Leuven, Belgium | CT DICOM data processing | |
| Materialise Mimics software version 25.1 | Materialise, Leuven, Belgium | Software to design the 3DHIP implant on the 3D model of the pelvis | |
| Implant manufacturer | |||
| Amnovis | Amnovis, Aarschot, Belgium | Printing and postprocessing of the 3DHIP implant. [https://www.amnovis.com/] | |
| Instrument and machine | |||
| 2.4 LeiLOX locking screw titanium | Rita Leibinger, BW, Germany | 242-224 | Titanium self tapping locking screw 2.4 mm. |
| 2.7 LeiLOX locking screw titanium | Rita Leibinger, BW, Germany | 242-227 | Titanium self tapping locking screw 2.7 mm. |
| 3.5 LeiLOX locking screw titanium | Rita Leibinger, BW, Germany | 242-235 | Titanium self tapping locking screw 3.5 mm. |
| BLUE SEAL 100 x 360 mm | Interster, Wormerveer, Netherlands | 3FKFB210819 | The transparent sterilization laminate size 100 x 360 mm |
| ETHILON 3-0 with FS-1 needle | Johnson & Johnson Medical GmbH, Norderstedt, Germany | 669H | Polyamide 6 3-0 (non-absorbable suture material) with 24 mm 3/8c reverse cutting needle using for skin closure |
| Fluoroscopy model NZS 229 | Philips, Eindhoven, Netherlands | Fluoroscopy | |
| Foley Catheter 10 fr x 90 cm (36") with 3 cc Balloon | MILA international inc., Kentucky, USA | MLIUC1036 | Foley urine catheter size 10 fr |
| Foley Catheter 6 fr x 60 cm (24") with 3 cc Balloon | MILA international inc., Kentucky, USA | MLIUC624 | Foley urine catheter size 6 fr |
| Foley Catheter 8 fr x 90 cm (36") with 3 cc Balloon | MILA international inc., Kentucky, USA | MLIUC836 | Foley urine catheter size 8 fr |
| Ioban 2 | 3M, MN, USA | 6640EU | Iodine-impregnated surgical drape |
| Miele professional G 7826 | Miele Nederland B.V., Vianen, Netherlands | The hygienic washing machine | |
| MMM sterilizer OB10643 | MMM Group, Planegg, Germany | Steam autoclave | |
| MONOCRYL 2-0 with SH Plus needle | Johnson & Johnson Medical GmbH, Norderstedt, Germany | MCP3170H | Poliglecaprone 25 plus antibacterial 2-0 (absorbable suture material) with 26 mm 1/2c taperpoint needle using for subcutaneous tissue closure |
| MONOCRYL 3-0 with SH Plus needle | Johnson & Johnson Medical GmbH, Norderstedt, Germany | MCP3160H | Poliglecaprone 25 plus antibacterial 3-0 (absorbable suture material) with 26 mm 1/2c taperpoint needle using for subcutaneous tissue closure |
| PDS 0 with CP needle | Johnson & Johnson Medical GmbH, Norderstedt, Germany | PDP485H | Polydioxanone plus antibacterial 0 (absorbable suture material) with 40 mm 1/2c reverse cutting needle using for muscle fascia and tendon closure |
| PDS 2-0 with CP-1 needle | Johnson & Johnson Medical GmbH, Norderstedt, Germany | PDP466H | Polydioxanone plus antibacterial 2-0 (absorbable suture material) with 36 mm 1/2c reverse cutting needle using for muscle fascia and tendon closure |
| ProX DMP320 | 3D systems, South Carolina, USA | Direct metal printing machine using selective laser melting technology | |
| Medications | |||
| Betadine oplossing | Mylan B.V., Amstelveen, Netherlands | RVG 01331 | Povidone-iodine solution 100 mg/mL (500 mL) |
| Betadine shampoo | Mylan B.V., Amstelveen, Netherlands | RVG 08943 | Povidone-iodine 75 mg/mL (120 mL) |
| Carporal 20 mg | AST Farma B.V. Oudewater, Netherlands | REG NL 101766 | Carprofen 20 mg/tablet |
| Carporal 40 mg | AST Farma B.V. Oudewater, Netherlands | REG NL 115715 | Carprofen 40 mg/tablet |
| Carporal 50 mg | AST Farma B.V. Oudewater, Netherlands | REG NL 101767 | Carprofen 50 mg/tablet |
| Cefazolin Mylan 1 g | Mylan B.V., Amstelveen, Netherlands | RVG 16532 | Cefazolin powder 1 g for injection |
| Chlorhexidine 0.5% in alcohol 70% spray | Orphi Farma BV, Lage Zwaluwe, Netherlands | 8711407672906 | Chlorhexidine 0.5% in alcohol 70% spray (250 mL) |
| Dexdomitor 0.5 mg/mL | Orion Corporation, Espoo, Finland | EU/2/02/033/001-002 | Dexmedetomidine hydrochloride 0.5 mg/mL for injection (20 mL) |
| Gabapentin Sandoz 300 mg | Sandoz B.V., Almere, Netherlands | RVG 33681 | Gabapentin 300 mg/capsule |
| GABAPENTINE TEVA 100 mg | Teva B.V., Haarlem, Netherlands | RVG 31980 | Gabapentin 100 mg/capsule |
| HiBiScrub | Mölnlycke Health Care AB., Utrecht, Netherlands | RVG 10156 | Chlorhexidine digluconate 40 mg/mL (500 mL) |
| Insistor 10 mg/mL | Richter pharma AG, Oostenrijk, Netherlands | REG NL 121166 | Methadone hydrochloride 10 mg/mL for injection (10 mL) |
| Isoflutek 1000 mg/g | Laboratorios Karizoo S.A., Barcelona, Spain | REG NL 118938 | Isoflurane 1000 mg/g (250 mL) |
| Levobupivacaine Fresenius Kabi 2.5 mg/mL | Fresenius Kabi Nederland b.v., Huis ter Heide, Netherlands | AWA 0611 | Levobupivacaine 2.5 mg/mL solution for injection (10 mL) |
| Morfine HCI CF 10 mg/mL | Centrafarm B.V., Breda, Netherlands | RVG 50836 | Morphine hydrochloride 10 mg/mL (1 mL) |
| Narketan 10 | Vetoquinol B.V., Breda, Netherlands | vm08007/4090 | Ketamine 10 mg/mL (10 mL) |
| Propofol 10 mg/mL | Fresenius Kabi Nederland b.v., Huis ter Heide, Netherlands | RVG 110627 | Propofol 10 mg/mL emulsion for injection or infusion (50 mL) |
| Rimadyl | Zoetis B.V., Capelle a/d Ijssel, Netherlands | REG NL 10101 | Carprofen 50 mL/mL for injection (20 mL) |
| Sufentanil-hameln 50 mcg/mL | Hameln pharma gmbh, Hameln, Germany | 4260016653249 | Sufentanil citrate 50 mcg/mL for injection |
| Trazadone EG 100 mg | EG (Eurogenerics) NV Heizel, Brussel, Belgium | BE439607 | Trazadone hydrochloride 100 mg/tablet |
References
- King, M. D. Etiopathogenesis of canine hip dysplasia, prevalence, and genetics. Vet Clin North Am Small Anim Pract. 47 (4), 753-767 (2017).
- Akis, I., et al. The association of genetic polymorphisms of bone formation genes with canine hip dysplasia. Iran J Vet Res. 21 (1), 40-45 (2020).
- Loder, R. T., Todhunter, R. J. The demographics of canine hip dysplasia in the United States and Canada. J Vet Med. 2017, 1-15 (2017).
- Schachner, E. R., Lopez, M. J. Diagnosis, prevention, and management of canine hip dysplasia: a review. Vet Med (Auck)l. 6, 181-192 (2015).
- Vezzoni, A., Boiocchi, S., Vezzoni, L., Vanelli, A. B., Bronzo, V. Double pelvic osteotomy for the treatment of hip dysplasia in young dogs). Vet Comp Orthop Traumatol. 23 (6), 444-452 (2010).
- Tavola, F., Drudi, D., Vezzoni, L., Vezzoni, A. Postoperative complications of double pelvic osteotomy using specific plates in 305 dogs. Vet Comp Orthop Traumatol. 35 (1), 47-56 (2022).
- Koch, D. A., Hazewinkel, H. A. W., Nap, R. C., Meij, B. P., Wolvekamp, W. T. C. Radiographic evaluation and comparison of plate fixation after triple pelvic osteotomy in 32 dogs with hip dysplasia. Vet Comp Orthop Traumatol. 06 (01), 09-15 (1993).
- Rose, S. A., Bruecker, K. A., Petersen, S. W., Uddin, N. Use of locking plate and screws for triple pelvic osteotomy. Vet Surg. 41 (1), 114-120 (2012).
- Remedios, A. M., Fries, C. L. Implant complications in 20 triple pelvic osteotomies. Vet Comp Orthop Traumatol. 06 (04), 202-207 (1993).
- Moses, P. A. Alternative surgical methods for treating juvenile canine hip dysplasia. Aust Vet J. 78 (12), 822-824 (2000).
- Harper, T. A. M. Femoral head and neck excision. Vet Clin North Am Small Anim Pract. 47 (4), 885-897 (2017).
- Forster, K. E., et al. Complications and owner assessment of canine total hip replacement: a multicenter internet based survey. Vet Surg. 41 (5), 545-550 (2012).
- Volstad, N. J., Schaefer, S. L., Snyder, L. A., Meinen, J. B., Sample, S. J. Metallosis with pseudotumour formation: Long-term complication following cementless total hip replacement in a dog. Vet Comp Orthop Traumatol. 29 (4), 283-289 (2016).
- Nesser, V. E., Kowaleski, M. P., Boudrieau, R. J. Severe polyethylene wear requiring revision total hip arthroplasty in three dogs. Vet Surg. 45 (5), 664-671 (2016).
- Verhoeven, G., Fortrie, R., Van Ryssen, B., Coopman, F. Worldwide screening for canine hip dysplasia: where are we now. Vet Surg. 41 (1), 10-19 (2012).
- Willemsen, K., et al. Patient-specific 3D-printed shelf implant for the treatment of hip dysplasia: Anatomical and biomechanical outcomes in a canine model. J Orthop Res. 40 (5), 1154-1162 (2021).
- Willemsen, K., et al. Patient-specific 3D-printed shelf implant for the treatment of hip dysplasia tested in an experimental animal pilot in canines. Sci Rep. 12 (1), 3032 (2022).
- Kwananocha, I., et al. Acetabular rim extension using a personalized titanium implant for treatment of hip dysplasia in dogs: short-term results. Front Vet Sci. 10, 1160177 (2023).
- Van Der Wal, B. C. H., Sakkers, R. J. B., Meij, B. P., Evers, L. A. M., Weinans, H. H. . Method of manufacturing an implant. EP3463198B1. , (2021).
- Brainard, B. M., Hofmeister, E. H. Anesthesia principles and monitoring. Small Animal Surgery. , (2012).
- Martinez-Taboada, F., Redondo, J. I. Comparison of the hanging-drop technique and running-drip method for identifying the epidural space in dogs. Vet Anaesth Analg. 44 (2), 329-336 (2017).
- Johnson, K. A. Approach to the craniodorsal aspect of the hip joint through a craniolateral incision in the dog. Piermattei’s Atlas of Surgical Approaches to the Bones and Joints of the dog and cat. , (2014).
- Whitelock, R. G., Dyce, J., Houlton, J. E. Repair of femoral trochanteric osteotomy in the dog. J Small Anim Pract. 38 (5), 195-199 (1997).
- Silveira, C. J., Saunders, W. B. Greater trochanter osteotomy as a component of cementless total hip replacement: Five cases in four dogs. Vet Surg. 51 (2), 303-310 (2022).
- Archibeck, M. J., Rosenberg, A. G., Berger, R. A., Silverton, C. D. Trochanteric osteotomy and fixation during total hip arthroplasty. J Am Acad Orthop Surg. 11 (3), 163-173 (2003).
- Vezzoni, A. Complications of double and triple pelvic osteotomies. Complications in Small Animal Surgery. , (2016).

