DNA Extraction and Comparison Between Old and Fresh Necrophilic Fly Samples
Summary
This article describes a protocol for fresh and old necrophilic fly DNA extraction using a modified common DNA extraction kit.
Abstract
A total of five samples of Chrysomya megacephala samples – three fresh samples, one sample stored in alcohol for 2 years, and one sample stored in dry sealed storage for 2 years protected from light only – were selected to investigate whether a blood DNA extraction kit could extract DNA from necrophilous flies and to determine whether alcohol could prolong the preservation of necrophilous flies' DNA. First, the blood DNA extraction kit was used to extract DNA from their thorax tissues. Then, the DNA purity and concentration were examined using a microplate reader and a fluorometer. Finally, PCR amplification and electrophoresis of the extracted DNA were done with necrophilic fly-specific primers located in the mitochondrial CO I gene sequence. The results showed that the DNA purity of all samples was greater than 2.0. The DNA concentration was observed to be of the following order: fresh samples > alcohol-preserved old samples > untreated, old samples. All samples had specific electrophoretic bands after PCR amplification. In conclusion, a blood DNA extraction kit can be used to extract DNA from necrophilic flies successfully, and the DNA concentration of fresh fly samples is greater than that of old fly samples. The flies can be stored in alcohol for a long time.
Introduction
The inference of the time of death has always been one of the key and difficult issues to be resolved in judicial practice. In the practice of forensic science, for a criminal case, determining postmortem interval plays a crucial role in deducing the time of the crime, locking in the suspect, and narrowing the scope of the investigation. Traditional methods of inferring the time of death are based primarily on early postmortem phenomena, which can generally only be used to infer the time of death within 24 h. However, the long time of death cannot be determined by postmortem phenomena. It is now generally recognized in the forensic science community that forensic entomology has the potential to be an effective method of inferring a longer time of death.
Necrophagous insects are named for their larvae that feed on corpses. Among them, whenever a corpse is present, the adult necrophilous flies will be the first to reach the corpse and lay their eggs or larvae. The larvae feed on corpse tissue and mature into pupae, which fledge into adults. Necrophilic flies play a huge role in the practice of forensic science because of their regular life cycle and geographic distribution, for example, deducting the time of death and determining the place of death1,2. However, the barrier to the use of necrophilous flies in forensic practice is species identification. Morphological methods are still used as the authoritative method for species identification of necrophilous flies. However, morphological species identification requires a high degree of insect integrity. If the flies were in different developmental stages, or due to morphological changes caused by environmental choices, those all make morphological examination more difficult. In particular, the morphology of pupae and pupal shells, which are most often found at the crime scene, is barely recognizable. This, together with the scarcity of morphological experts, makes huge difficulty to the identification of species morphologically. Therefore, the application of molecular biology methods for species identification of flies has emerged, which reduces the difficulty of morphological identification and is independent of developmental stage3,4,5,6,7.
The first step in species identification of necrophilous flies based on molecular biology methods is the efficient extraction of DNA, while specialized kits for insect DNA extraction are not commonly available currently. And how to use common blood kits for DNA extraction of necrophilic flies becomes more forensically relevant. Necrophilic flies found at crime scenes are often mutilated or have undergone decay and DNA degradation. Fly samples are not immediately available for DNA extraction after acquisition, or if possible, complete samples need to be retained due to evidence preservation requirements. Based on the above requirements, in this study, we applied the blood DNA kit based on proteinase K digestion, and selected three fresh Chrysomya megacephala (Diptera, Lepidoptera) samples (Figure 1), one old C. megacephala sample placed in alcohol for two years, and one C. megacephala sample placed in light-protected storage for two years, weighed each of the five samples, elected the insect thorax tissue for DNA extraction. Comparing the quality and purity of DNA extracted from the old and new samples, PCR amplification and electrophoresis of the extracted DNA were performed with necrophilic fly-specific primers located in the mitochondrial CO 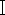 gene sequence.
gene sequence.
Protocol
Representative Results
Discussion
DNA extraction is the most critical link in the molecular identification of necrophilous flies, and its extraction method and the quality of the extracted DNA directly affect subsequent detection. In this experiment, we successfully extracted DNA from necrophilous flies by using a common blood kit, without the need to purchase a special insect DNA extraction kit, which makes insect DNA extraction easier to accomplish.
The surface of flies is rich in chitin, especially in the larval and pupal s…
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (82060341,81560304) and by the Academician Innovation Platform Scientific Research Project of Hainan Province (YSPTZX202134).
Materials
| Buffer AE | Qiagen | 172026832 | DNA elution buffer |
| Buffer AL | Qiagen | 172028374 | lysis buffer |
| Buffer ATL | Qiagen | 172028162 | tissue lysis buffer |
| Buffer AW1 | Qiagen | 172028760 | protein removal buffer |
| Buffer AW2 | Qiagen | 57203108 | desalination buffer |
| C1-J-2495 | Taihe Biotechnology | TW21109216 | forword primer |
| C1-N-2800 | Taihe Biotechnology | TW21109217 | reverse primer |
| D2000 DNA ladder | Real-Times(Beijing) Biotechnology | RTM415 | Measure the position of electrophoretic bands |
| DNeasy Mini spin column | Qiagen | 166050343 | DNA adsorption column |
| Dry Bath Incubator | Miulab | DKT200-2D | used for heating |
| MIX-30S Mini Mixer | Miulab | MUC881206 | oscillatory action |
| Proteinase K | Qiagen | 172026218 | Inactivation of intracellular nucleases and other proteins |
| Qubit 3.0 Fluorometer | Thermo Fisher Scientific | 2321611188 | |
| Speed Micro-Centrifuge | Scilogex | 9013001121 | centrifuge |
| Standard#1 | Thermo Fisher Scientific | 2342797 | |
| Standard#2 | Thermo Fisher Scientific | 2342797 | |
| Tanon 3500R Gel Imager | Tanon | 16T5553R-455 | gel imaging |
| Taq Mix Pro | Monad | 00007808-140534 | PCR Mix |
| Taq Mix Pro | Monad | 00007808-140534 | PCR Mix |
| Thermo Cycler | Zhuhai Hema | VRB020A | ordinary PCR |
| Working Solution | Thermo Fisher Scientific | 2342797 |
References
- Amendt, J., Richards, C. S., Campobasso, C. P., Zehner, R., Hall, M. J. Forensic entomology: applications and limitations. Forensic Sci Med Pathol. 7 (4), 379-392 (2011).
- Wydra, J., Matuszewski, S. The optimal post-eclosion interval while estimating the post-mortem interval based on an empty puparium. Forensic Sci Med Pathol. 17 (2), 192-198 (2021).
- Mazzanti, M., Alessandrini, F., Tagliabracci, A., Wells, J. D., Campobasso, C. P. DNA degradation and genetic analysis of empty puparia: genetic identification limits in forensic entomology. Forensic Sci Int. 195 (1-3), 99-102 (2010).
- Odeniran, P. O., et al. molecular identification and distribution of Trypanosome-transmitting Dipterans from cattle settlements in Southwest Nigeria. Acta Parasitol. 66 (1), 116-128 (2021).
- Wells, J. D., Stevens, J. R. Application of DNA-based methods in forensic entomology. Annu Rev Entomol. 53, 103-120 (2008).
- Kotrba, M., et al. DNA barcoding in forensic entomology-establishing a DNA reference library of potentially forensic relevant arthropod species. J Forensic Sci. 64 (2), 593-601 (2019).
- Weedon, M. N., et al. Use of SNP chips to detect rare pathogenic variants: Retrospective, population based diagnostic evaluation. BMJ. 372, 214 (2021).
- Cai, J. -. F., et al. The availability of mitochondrial DNA cytochrome oxidasegene for the distinction of forensically important flies in China. Acta Entomologica Sinica. 48 (3), 380-385 (2005).
- Khayrova, A., Lopatin, S., Varlamov, V. Obtaining chitin, chitosan and their melanin complexes from insects. Int J Biol Macromol. 167, 1319-1328 (2021).
- Guo, Y. Q., et al. Comparison of three DNA extracting methods from different parts of common necrophagous flies. J Henan Univ Sci Tech (Med Sci). 36 (02), 130-133 (2018).
- Du, H., Xie, H., Ma, M., Igarashi, Y., Luo, F. Modified methods obtain high-quality DNA and RNA from anaerobic activated sludge at a wide range of temperatures. J Microbiol Methods. 199, 106532 (2022).
- Sukumaran, S. Concentration determination of nucleic acids and proteins using the micro-volume BioSpec-nano-spectrophotometer. J Vis Exp. (48), e2699 (2011).
- Masago, K., et al. Comparison between fluorimetry (Qubit) and spectrophotometry (NanoDrop) in the quantification of DNA and RNA extracted from frozen and FFPE tissues from lung cancer patients: A real-world use of genomic tests. Medicina (Kaunas). 57 (12), 1375 (2021).
- Ponti, G., et al. The value of fluorimetry (Qubit) and spectrophotometry (NanoDrop) in the quantification of cell-free DNA (cfDNA) in malignant melanoma and prostate cancer patients. Clin Chim Acta. 479, 14-19 (2018).
- Adams, Z. J., Hall, M. J. Methods used for the killing and preservation of blowfly larvae, and their effect on post-mortem larval length. Forensic Sci Int. 138 (1-3), 50-61 (2003).
- Fliegerova, K., et al. Effect of DNA extraction and sample preservation method on rumen bacterial population. Anaerobe. 29, 80-84 (2014).
- Rodrigues, B. L., Galati, E. A. B. Molecular taxonomy of phlebotomine sand flies (Diptera, Psychodidae) with emphasis on DNA barcoding: A review. Acta Trop. 238, 106778 (2023).

.
