- 00:00Overview
- 01:14Principles of Measuring Dissolved Oxygen in Surface Water
- 03:27Sample Collection and Fixing in the Field
- 04:42Measuring Dissolved Oxygen in Surface Water Samples in the Laboratory
- 06:04Results
- 07:01Applications
- 09:07Summary
Oxygène dissous dans les eaux de surface
English
Share
Overview
Source : Laboratoires de Margaret Workman et Kimberly Frye – Depaul University
Oxygène dissous (DO) mesures de calculer la quantité d’oxygène gazeux dissous dans les eaux de surface, ce qui est important pour toute la vie respirant de l’oxygène dans les écosystèmes du fleuve, y compris les espèces de poisson préférées pour la consommation humaine (p. ex. crapet arlequin et basse), ainsi que des espèces de décomposeurs critiques pour le recyclage de matériaux biogéochimiques dans le système.
L’oxygène dissous dans les lacs, rivières et Océans est cruciale pour les organismes et les créatures qui vivent dedans. Que la quantité d’oxygène dissous tombe au-dessous des niveaux normaux en plans d’eau, la qualité de l’eau est bafouée et créatures commencent à mourir. Dans un processus appelé eutrophisation, un plan d’eau peut devenir hypoxique et ne sera plus en mesure d’appuyer les organismes vivants, essentiellement devenir une « zone morte ».
L’eutrophisation se produit lorsque les nutriments en excès provoquent des populations d’algues à croître rapidement dans une prolifération des algues. Les efflorescences algales forme des tapis denses à la surface de l’eau en bloquant deux intrants essentiels de l’oxygène de l’eau : gaz change de l’atmosphère et la photosynthèse dans l’eau en raison du manque de lumière en dessous du tapis. Comme l’oxygène dissous niveaux diminuent au-dessous de la mortalité des organismes surface, respirant de l’oxygène en grande quantité, créant une augmentation en matière organique. Les causes de l’excès de matière organique une augmentation dans les populations de décomposeurs respirant de l’oxygène dans la zone benthique, qui outre épuise l’oxygène dissous restant niveaux durant l’activité métabolique de la décomposition. Dès que les niveaux d’oxygène devient cette espèce de respiration d’oxygène faible et mobile (p. ex. poissons) va s’éloigner, laissant sans vie aérobie dans l’eau et la création d’une zone morte.
La méthode de titration de l’azoture-Winkler utilise titrage pour déterminer la concentration d’un inconnu dans un échantillon. Plus précisément, du thiosulfate de sodium est utilisé pour titrer l’iode, qui peut être stoechiométriquement reliée à la quantité d’oxygène dissous dans un échantillon.
Principles
Procedure
Results
A dissolved oxygen level of 6 mg/L is sufficient for most aquatic species. Dissolved oxygen levels below 4 mg/L are stressful to most aquatic animals. Dissolved oxygen levels below 2 mg/L will not support aerobic aquatic life (Figure 5).
The maximum amount of oxygen that can be dissolved in water varies by temperature (Table 1).
DO measurements in mg/L are converted to % saturation using water temperature and the conversion chart below (Figure 6).
DISSOLVED OXYGEN LEVELS (% SATURATION)
Excellent: 91 – 110
Good: 71 – 90
Fair: 51 – 70
Poor: < 50
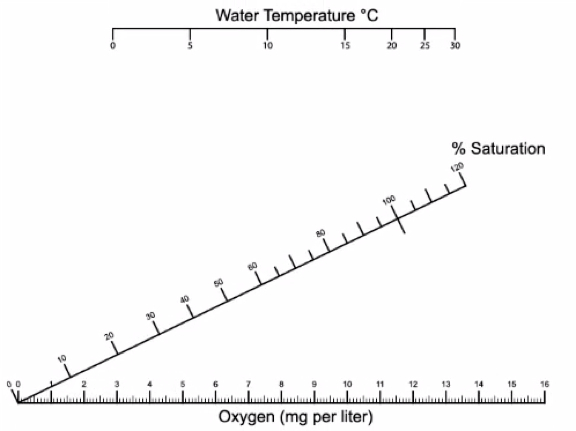
Figure 5. DO measurements are converted to % saturation using the water’s temperature. The water’s temperature on the top horizontal axis and the measured DO value on the bottom horizontal axis. Use a ruler to draw a line between the two values and record where the line meets the middle diagonal axis for % saturation.
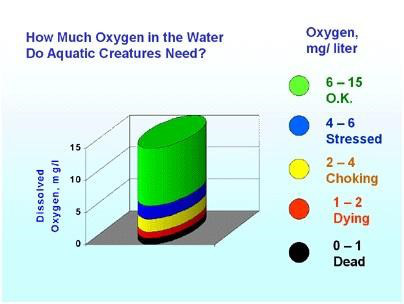
Figure 6. A dissolved oxygen level of 6 mg/L is sufficient for most aquatic species. Dissolved oxygen levels below 4 mg/L are stressful to most aquatic animals. Dissolved oxygen levels below 2 mg/L will not support fish and below 1 mg/L will not support most species.
| Temp. (°C) | DO (mg/L) | Temp. (°C) | DO (mg/L) | Temp.(°C) | DO (mg/L) | Temp.(°C) | DO (mg/L) |
| 0 | 14.60 | 11 | 11.01 | 22 | 8.72 | 33 | 7.16 |
| 1 | 14.19 | 12 | 10.76 | 23 | 8.56 | 34 | 7.16 |
| 2 | 13.81 | 13 | 10.52 | 24 | 8.40 | 35 | 6.93 |
| 3 | 13.44 | 14 | 10.29 | 25 | 8.24 | 36 | 6.82 |
| 4 | 13.09 | 15 | 10.07 | 26 | 8.09 | 37 | 6.71 |
| 5 | 12.75 | 16 | 9.85 | 27 | 7.95 | 38 | 6.61 |
| 6 | 12.43 | 17 | 9.65 | 28 | 7.81 | 39 | 6.51 |
| 7 | 12.12 | 18 | 9.45 | 29 | 7.67 | 40 | 6.41 |
| 8 | 11.83 | 19 | 9.26 | 30 | 7.54 | 41 | 6.41 |
| 9 | 11.55 | 20 | 9.07 | 31 | 7.41 | 42 | 6.22 |
| 10 | 11.27 | 21 | 8.90 | 32 | 7.28 | 43 | 6.13 |
Table 1. Maximum amounts of oxygen that can be dissolved in water by temperature.
Applications and Summary
Slow-moving rivers are particularly vulnerable to low DO levels, and in extreme cases, these DO levels can lead to hypoxic conditions, creating “dead zones” where aerobic life is no longer supported by a body of water (Figure 7). Once plants and animals die-off, the build-up of sediment that occurs can also raise the riverbed, allowing plants to colonize over the water and could lead to the loss of the river all together (Figure 8). Surface waters at higher altitudes are also more vulnerable to low DO levels, as atmospheric pressure decreases with increasing altitude, and less oxygen gas is suspended in the water.
Low DO levels support life forms considered unappealing or unfit for human use, including leeches and aquatic worms (Oligochaeta).
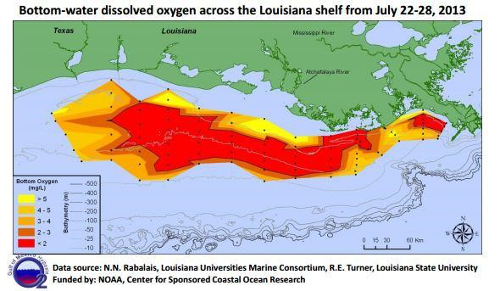
Figure 7. Map of dissolved oxygen concentrations across the Louisiana shelf showing the dead zone region.
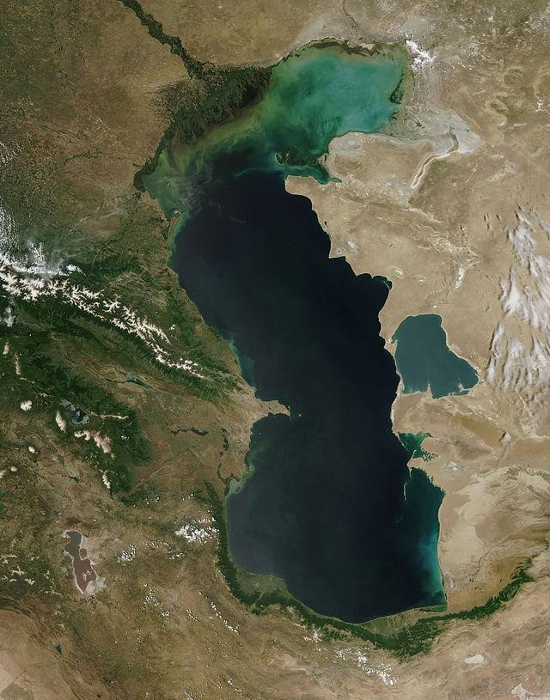
Figure 8. Photograph of the Caspian Sea showing severe eutrophication in the north end.
Transcript
Dissolved oxygen is crucial for river and lake ecosystems to support aerobic life. The Azide-Winkler titration method allows quantification of the amount of dissolved oxygen in surface water samples.
Gaseous oxygen dissolved in surface water is required for the survival of the organisms living in it; decomposers critical to recycling of biogeochemical materials in the ecosystem, or fish species preferred for human consumption. As oxygen levels fall below normal in water systems, water quality is harmed and organisms begin to die.
The Azide-Winkler titration method is a standard test to determine the concentration of dissolved oxygen in a sample. Sodium thiosulfate is used to titrate iodine, which is stochiometrically related to the amount of dissolved oxygen in the sample.
This video will illustrate the principles behind dissolved oxygen quantification, the process of performing the Azide-Winker titration, and the interpretation of dissolved oxygen measures.
Eutrophication is the introduction of excess nutrients into an ecosystem. This causes algae populations to grow rapidly into dense mats, known as algal blooms. These mats can lead to hypoxia, or low oxygen levels, by blocking out gas exchange at the surface, and prevent photosynthesis by blocking sunlight. Oxygen breathing organisms begin to die, causing an increase in organic matter, which in turn causes an increase in oxygen dependent decomposers, depleting oxygen resources yet further. Finally, mobile oxygen-dependent organisms move away, leaving a dead zone with no aerobic life.
To test the level of dissolved oxygen in a water source, the Azide-Winkler method can be used to measure dissolved oxygen directly in the field, or samples can be fixed and taken to the laboratory for further analysis.
Manganese sulfate and potassium hydroxide are added to the sample, forming manganese hydroxide. This reduces the dissolved oxygen, forming a brown precipitate. Alkaline iodide-azide reagent is added to correct for the presence of nitrates found in wastewater samples that can interfere with the oxidation procedure.
Added sulfuric acid acidifies the solution and dissolves the precipitate. This new compound oxidizes the iodide from the alkaline iodine-azide reagent to iodine.
Next, a starch indicator is added that will turn blue in the presence of iodine. Thiosulfate, which turns iodine back into iodide, is used to titrate the iodine. When the titration is complete, the blue solution will turn colorless. The amount of dissolved oxygen in the sample is proportional to the amount of thiosulfate required to turn the solution from blue to colorless.
Now that we are familiar with the principles behind measuring dissolved oxygen in water samples, let’s take a look at how this is carried out in the field and the laboratory.
The experiment will begin at the collection site. First, collect the sample water in a clear 300-mL BOD bottle. Next, measure and record the temperature of water from the water source. Carefully add 2 mL manganous sulfate to the sample by inserting the pipette tip under the water surface and slowly dispense to avoid creating bubbles.
Using the same technique, add 2 mL alkaline iodine-azide reagent, and immediately insert the stopper, tilting the bottle slightly so no air is trapped in the bottle.
Carefully invert several times to mix the solution, taking care not to create air bubbles. A precipitate will form, causing a cloudy appearance. Let the precipitate in the solution settle, and then mix thoroughly by inverting the bottle several times before letting it settle again. Samples should be sealed using a small amount of deionized water squirted around the stopper, then wrapped in aluminum foil and secured with a rubber band. The sample is now fixed, and can be transported back to the laboratory.
Once the samples have been fixed, they are transported to the lab for further analysis. First, holding the pipette tip just above the sample surface, add 2 mL of concentrated sulfuric acid into the sample. Invert several times to dissolve the precipitate. Using a glass flask and calibrated pipette, titrate 200 mL of the pre-treated sample water with 0.025 N standardized sodium thiosulfate, swirling and mixing continuously until a pale straw color forms.
Once the solution is straw colored, add 2, 1-mL droplets of starch indicator solution and swirl to mix. The solution will turn blue. Continue the titration, adding one drop of sodium thiosulfate at a time and mixing slowly using a stir bar until the blue dissipates and the solution becomes colorless. Hold the sample against a white piece of paper to enhance visualization. Record the volume of thiosulfate added.
The concentration of dissolved oxygen is proportional to the volume of sodium thiosulfate added to the sample. Each milliliter added is equivalent to 1 mg/L, or parts per million, dissolved oxygen.
The maximum amount of oxygen that can be dissolved in water varies by water temperature. Dissolved oxygen measurements in mg/L are converted to percent saturation using water temperature and a conversion chart. Saturation of 91 to 110% dissolved oxygen is considered excellent; between 71 and 90% is good, 51-70% is fair, and below 50% is poor.
Dissolved oxygen levels of 6 mg/L are sufficient to support most aquatic species. Levels below 4 mg/L are stressful to the majority of aquatic animals, so biodiversity will be affected. Water containing less than 2 mg/L dissolved oxygen will not support aerobic aquatic life.
The ability to quantify the amount of dissolved oxygen in a water source also has alternative methods, and many relevant practical applications. Some of these are explored here.
Dissolved oxygen and temperature can also be measured using a handheld LabQuest monitor with dissolved oxygen and temperature probes. For dissolved oxygen, plug the probe into channel 1. Units should be in mg/L. Submerge the probe into the water sample, circulating the probe slowly through the sample to avoid consuming oxygen in a localized area. When the readings appear to stabilize, record the value.
Most fish require moderate to good levels of dissolved oxygen in their habitats to thrive and reproduce. For fish farms, which may occupy man-made or natural lakes or streams, being able to test dissolved oxygen levels can help farm managers to choose a good initial set-up site, or to keep track of the health of their pools or streams.
Monitoring dissolved oxygen can also be useful for habitat management and conservation. If a lake or river region contains protected or endangered flora or fauna, monitoring of dissolved oxygen levels can give an indication of the health of the ecosystem. If levels change rapidly, this could indicate danger for the protected species, and may indicate that a management intervention strategy should be implemented.
The United States Environmental Protection Agency, the EPA, suggests a number of measures to correct dissolved oxygen levels in ecosystems. These include correct and minimal use of fertilizers, proper wastewater treatment, not discharging sewage from boats, and preserving adjoining rivers, streams, and wetlands. Reducing nitrogen oxides by minimizing electricity and automobile use and choosing more efficient boat engines can also help to maintain appropriate dissolved oxygen levels in water resources.
You’ve just watched JoVE’s introduction to measuring dissolved oxygen in surface waters. You should now understand the principles behind dissolved oxygen measurement, how to quantify dissolved oxygen in your own water samples, and how to interpret your findings and their implications for the environment. Thanks for watching!



