エバンス メソッド
English
Share
Overview
ソース: タマラ ・ m ・力、化学科テキサス A & M 大学
ほとんどの有機性分子は反磁性、前記、債券、多くの遷移金属錯体の電子がペアになりすべてが常磁性、地面のある状態不対電子のです。リコール フントの規則は、似たようなエネルギーの軌道の電子が埋めるを組み合わせる前に不対電子の数を最大化する軌道の状態します。遷移金属が部分的にdを設定-軌道のエネルギーが金属に配位子の調整によって様々 な範囲に摂動します。したがって、 d-軌道はエネルギー、互いに似ていますが、すべて縮退はないです。これにより、ペアすべての電子、反磁性または常磁性、不対電子を持つ錯体。
金属の複合体中の不対電子の数を知ることは、配位子の配位子 (クリスタル フィールド) 電界と同様、酸化状態と金属錯体のジオメトリに手がかりを提供できます。これらのプロパティは大きく分光と遷移金属錯体の反応性に影響を与えるし、理解することが重要。
不対電子の数をカウントする方法の 1 つは、 χ、配位化合物の磁気感受性を測定することです。帯磁は材料 (または複合) ときの磁化の測定応用磁気フィールドに配置されます。対電子は磁場に少し反発、この反発は、磁場の増加の強さと比例して増加します。その一方で、不対電子は磁界を (より大きい範囲) に引き付けられるし、魅力は、磁場強度に比例して増加します。したがって、不対電子を持つ任意の化合物は、磁場に魅了されるでしょう。1
磁化率測定、我々 は磁気モーメント、μ から不対電子の数に関する情報を取得します。帯磁は磁気モーメント、方程式 12: μ に関連して
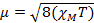 (1)
(1)
定数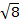 = [(3 kB)/Nβ2)]、β = 電子 (0.93 x 10-20 erg ガウス-1)、N のボーア磁子 = Avogadro の数、kB = ボルツマン定数
= [(3 kB)/Nβ2)]、β = 電子 (0.93 x 10-20 erg ガウス-1)、N のボーア磁子 = Avogadro の数、kB = ボルツマン定数
M x = モル磁化率 (cm3/mol)
T = 温度 (K)
μ = μBボーア磁子の単位で測定される磁気モーメント = 10-24 JT-1 x 9.27
錯体の磁気モーメント方程式 21:により与えられる
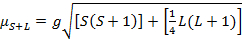 (2)
(2)
g gyromagnetic 比を = = 2.00023 μB
S = スピン量子数 = ∑ms = [不対電子、 nの数]/2
L = 軌道量子数= ∑ml
この式は、軌道とスピンの両方の貢献です。最初の行遷移金属錯体の軌道の寄与が小さく、したがって省略できます、スピンだけの磁気モーメントにより与えられるので式 3:
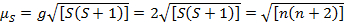 (3)
(3)
直接、スピンだけの磁気モーメントでは、不対電子の数を与えることができる従って。この近似が可能重い金属の軌道の貢献は 2 番目と 3 番目の行の遷移金属のために重要かもしれないが。この貢献は、それが化合物より対になっていない電子は、それよりも、十分に磁気モーメントを膨らませますので重要な可能性があります。したがって、さらに特性評価は、これらの複合体に要求されるかもしれない。
この実験では tris(acetylacetonato)iron(III) (Fe(acac)3) のソリューションの磁気モーメントは実験クロロホルム ・ エヴァンス メソッドを使用して決定されます。
Principles
Procedure
Results
Experimental Results
| Fe(acac)3 | Chloroform | |
| m (g) | 0.0051 | 0.874 |
| MW (g/mol) | 353.17 | n/a |
| n (mol) | 1.44⋅10–5 | n/a |
| Density (g/mL) | n/a | 1.49* |
| Volume (mL) | n/a | 0.587 |
| c (mol/mL) | 2.45⋅10-5 | |
| NMR shifts | Peak 1 | Peak 2 |
| δ (ppm) | 7.26 | 5.85 |
| Δppm | 1.41 | |
| NMR Instrument | ||
| Temperature (K) | 296.3 | |
| Field, F (Hz) | 500⋅106 |
* the density of the solvent can be approximated to the density of the solvent used
Calculations:
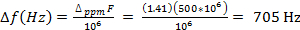
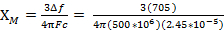 = 0.0137 cm3/mol
= 0.0137 cm3/mol
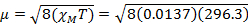 = 5.70 µB
= 5.70 µB
Theoretical Results for Given S and n Values:
| S | n | μS |
| 1/2 | 1 | 1.73 |
| 1 | 2 | 2.83 |
| 3/2 | 3 | 3.87 |
| 2 | 4 | 4.90 |
| 5/2 | 5 | 5.92 |
For 4.5 mg of Fe(acac)3 dissolved in 0.58 mL solvent, with a 300 MHz instrument a peak separation of 1.41 ppm is observed, which gives XM= 1.37 x 10-2 and µeff = 5.70. This µeff value is consistent with an S = 5/2 complex, which has 5 unpaired electrons.
Applications and Summary
The Evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. This provides the number of unpaired electrons in a metal complex, which is pertinent to the spectroscopy, magnetic properties, and reactivity of the complex.
Measuring the magnetic susceptibility of paramagnetic species gives the number of unpaired electrons, which is a key property of metal complexes. As the reactivity of metal complexes is influenced by its electronic structure – that is, how the d-orbitals are populated – it is important to establish the number of unpaired electrons. The magnetic susceptibility can be used to determine the geometry of the metal complex in solution, give insight into the ligand field strength, and can provide evidence for the correct formal oxidation-state assignment of the metal complex. In the modules on "Group Theory" and "MO Theory of Transition Metal Complexes," we will introduce how to predict d-orbital splitting diagrams as well as how to use data from the Evans method to help determine the geometry of a metal complex and provide evidence for the oxidation state of the metal center.
There are multiple instruments that can be used to measure the magnetic susceptibility of a paramagnetic species including a Gouy balance, SQUID, or NMR instrument. The Evans method is a simple and practical technique that uses NMR to determine the solution magnetic moment of a paramagnet. While the Evans method is a powerful tool in the field of magnetism, there are several drawbacks to the technique. First, the molecule must be soluble in the solvent used in the experiment. If the paramagnetic sample is not fully dissolved, the concentration of the solution will be incorrect, which will lead to errors in the experimentally determined solution magnetic moment. Other errors in concentration can arise if the paramagnetic sample has diamagnetic (solvent) or paramagnetic impurities.
References
- Miessler, G. L., Fischer, P. J., Tarr, D. A. Inorganic Chemistry. 5 ed. Pearson. (2014).
- Drago, R. S. Physical Methods for Chemists. 2 ed. Saunders College Publishing. (1992).
- Girolami, G. S., Rauchfuss, T. B., Angelici, R. J. Synthesis and Technique in Inorganic Chemistry: A Laboratory Manual. 3 ed. University Science Books. Sausalito, CA, (1999).
Transcript
The Evans method is a technique for calculating the number of unpaired electrons in solution-state metal complexes.
Many transition metal complexes have unpaired electrons, making them attracted to magnetic fields. These complexes are called paramagnetic. Complexes with all paired electrons are called diamagnetic.
Knowing the number of unpaired electrons is important for predicting the reactivity of a compound. The Evans method uses NMR spectroscopy to measure the parameters needed to calculate the number of unpaired electrons.
This video will illustrate the procedure for performing the Evans method, demonstrate the analysis of Fe(acac)3, and introduce a few applications of counting unpaired electrons in chemistry.
The number of unpaired electrons in a complex can be determined from the magnetic moment of the given molecule. The magnetic moments of 1st row transition metal complexes can be approximated from the contributions of unpaired electrons, called the spin-only magnetic moment. For the 2nd and 3rd row transition metal complexes, both the spin and orbital contributions must be considered.
The magnetic moment is related to the magnetic susceptibility, which provides the degree of magnetization of a complex in an applied magnetic field.
The chemical shift of a species in an NMR spectrum is affected by the overall magnetic susceptibility of the sample solution. Thus, the chemical shift of a solvent changes if the solute is paramagnetic. The Evans method uses this relationship to obtain the magnetic susceptibility, and thus the magnetic moment, of that paramagnetic solute.
An Evans method sample uses a capillary insert containing a mixture of a deuterated solvent and the matching proteated solvent. The compound of interest is dissolved in the same solvent mixture and placed in an NMR tube with the capillary.
The acquired NMR spectrum shows two solvent peaks: one corresponding to the proteated solvent in solution with the compound, and the other corresponding to the proteated solvent in the capillary.
The magnetic susceptibility is calculated from the frequency difference and the concentration of the paramagnetic compound in the sample.
The magnetic moment is calculated from the magnetic susceptibility in a special unit called the Bohr magneton. The magnetic moment can then be compared to theoretical spin-only values to estimate the number of unpaired electrons in the sample.
Now that you understand the principles of the Evans method, let’s go through a procedure for finding the number of unpaired electrons in Fe(acac)3 with the Evans method.
To prepare the capillary insert, melt the tip of a long Pasteur pipette with a flame until the tip melts into a glass bulb. Allow the glass to cool.
Next, combine in a clean scintillation vial 2 mL of a deuterated solvent and 40 μL of a proteated solvent. Cap the vial and swirl gently.
Carefully add a few drops of the solvent mixture to the cooled pipette. Gently flick or tap the pipette tip until the solvent has gathered at the bottom of the tip.
Continue adding the solvent mixture in this way until the solution fills the sealed pipette tip to a depth of about 2 inches, with no air bubbles.
Cap the pipette with a 14/20 rubber septum. Equip a 3-mL syringe with a needle. Insert the needle through the septum and carefully withdraw 3 mL of air.
Remove the syringe and clamp the pipette to a ring stand horizontally. Use a lighter to soften the glass above the solution in the pipette tip.
Once the glass begins softening, slowly rotate the solution-filled pipette tip to seal in the solution. Continue rotating the newly-formed capillary until it easily separates from the pipette body.
Let the capillary insert cool, and then store it in a labeled container.
To prepare a sample for the Evans method, first record the mass of a scintillation vial and cap. Then, place 5 mg of the paramagnetic compound of interest into the scintillation vial and record the mass.
Pipette about 600 μL of the mixture of deuterated and proteated solvents into the scintillation vial. Swirl the vial until the solid compound completely dissolves.
Record the mass of the capped vial of sample solution. Then, obtain a standard NMR tube and cap.
Carefully slide the capillary insert into the NMR tube at an angle. Transfer the solution of the paramagnetic compound to the NMR tube and cap the tube. Ensure that the insert is sitting at the bottom of the tube.
Acquire and save a standard 1H NMR spectrum.
First, calculate the concentration of the sample solution in moles per cubic centimeter using the recorded masses and the density of the solvent. Then, convert the difference between the solvent peak chemical shifts from ppm to Hz. Calculate the molar magnetic susceptibility of the sample.
Next, calculate the magnetic moment from the probe temperature and the molar magnetic susceptibility. Compare the calculated value with a table of known values to determine the number of unpaired electrons in the compound.
The number of unpaired electrons is important for modeling chemical and biological complexes. Let’s look at a few applications.
Transition metal complexes can be modeled with molecular orbital theory. In this model, electrons are assigned to molecular orbitals shared between atoms. Information about the number of unpaired electrons helps to confirm that an appropriate model is being used. Further, the number of singly-occupied and unoccupied orbitals predicts how the complex will react with other molecules.
Molecules can be classified by the symmetry operations that they can perform, such as being mirrored across an axis. Molecular symmetry can predict many properties, such as the vibrational modes of a compound. As the number of unpaired electrons can provide information about molecular geometry, it is important to accurately determine the number of unpaired electrons when characterizing compounds.
You’ve just watched JoVE’s introduction to the Evans method. You should now understand the underlying principles of the Evans method, the procedure for calculating the number of unpaired electrons, and how unpaired electrons are relevant to understanding chemical reactivity. Thanks for watching!
