טומוגרפיה פוטואקוסטית לדם תמונה ולומנים בבטורה האינפרא-רנאלית
English
Share
Overview
מקור: גורנייט ס. סנגה וקרייג ג’יי גורגן, בית הספר להנדסה ביו-רפואית של ולדון, אוניברסיטת פרדו, מערב לאפייט, אינדיאנה
טומוגרפיה פוטואקוסטית (PAT) היא שיטת הדמיה ביו-רפואית מתפתחת המשתמשת בגלים אקוסטיים שנוצרו על ידי אור כדי לקבל מידע קומפוזיציה מרקמות. PAT יכול לשמש כדי לדמיין דם ורכיבי שומנים, אשר שימושי עבור מגוון רחב של יישומים, כולל הדמיה לב וכלי דם וגידול. לטכניקות הדמיה הנמצאות בשימוש כיום יש מגבלות מובנות המגבילות את השימוש בהן עם חוקרים ורופאים. לדוגמה, זמני רכישה ארוכים, עלויות גבוהות, שימוש בניגודיות מזיקה ומינימום פולשניות הם כל הגורמים המגבילים את השימוש במודלים שונים במעבדה ובמרפאה. נכון לעכשיו, טכניקות ההדמיה הדומות היחידות ל- PAT הן טכניקות אופטיות מתפתחות. אבל אלה יש גם חסרונות, כגון עומק מוגבל של חדירה ואת הצורך סוכני ניגוד אקסוגני. PAT מספק מידע משמעותי באופן מהיר, לא פולשני, ללא תוויות. כאשר בשילוב עם אולטרסאונד, PAT יכול לשמש כדי לקבל מידע מבני, hemodynamic, קומפוזיציה מן הרקמה, ובכך להשלים טכניקות הדמיה בשימוש הנוכחי. היתרונות של PAT ממחישים את היכולות שלה להשפיע הן בסביבה פרה-קלינית והן בסביבה הקלינית.
Principles
Procedure
Results
Here, VPAT methods were used to perform lipid and blood specific imaging in vivo. By coupling a laser and ultrasound system, light was delivered to tissue and the resulting acoustic waves were detected. Ultrasound imaging allowed us to obtain structural information of the infrarenal aorta (Figure 1a) that can be used to better interpret VPAT compositional information. Specifically, a 1100 nm light was used to image blood within the aorta (Figure 1b), and a 1210 nm light was used to image subcutaneous and periaortic fat accumulation (Figure 1c). From the ultrasound and VPAT images, one can see that the subcutaneous fat follows the geometry of the skin, the periaortic fat follows the contour of the aorta, and the blood signal originates from within the aorta. These results confirm that, indeed, VPAT can be used to image blood and lipid accumulation in vivo.
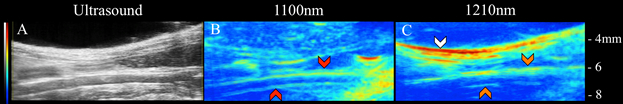
Figure 1: Ultrasound (left), blood VPAT (middle), and lipid VPAT (right) images of ApoE-/-. The subcutaneous fat (white arrows), periaortic fat (orange arrows), and blood (red arrows) is clearly visible.
Applications and Summary
VPAT is a rapid, noninvasive, label-free method to image blood and lipid accumulation in vivo. By delivering pulsed laser light to tissue, acoustic propagations were induced to obtain relative density and locate biological components. When coupled with ultrasound imaging, compositional, as well as structural and hemodynamic information from tissue, can be resolved. A current limitation of this technique is its penetration depth, which is roughly 3 mm for lipid-based imaging. While this is better than current optical techniques, improvements to light delivery techniques would improve the depth of penetration. One way to improve this is by developing a photoacoustic transducer that maximizes light delivery to region of interest while redirecting reflected light back into tissue. While VPAT is an imaging technique that is still in its infancy, it has received a great deal of interest in recent years, making it likely that this technique will be used in more laboratories and clinics in the future.
The described protocol can be used for a wide variety of applications in both the preclinical and clinical space. Three potential VPAT applications include utilizing the technique to 1) study lipid-based disease progression, 2) evaluate promising therapeutics, and 3) improve diagnosis of lipid-based diseases. The capability of tracking structural, hemodynamic, and compositional information makes VPAT an appealing technology to study how vascular lipid accumulates in small animals models (Figure 1). Moreover, since VPAT is a noninvasive method it can be applied to evaluate the effects of therapeutics in longitudinal studies. This could specifically lower the cost of research by decreasing the number of animals needed for therapy validation. Finally, the ability of VPAT to provide compositional information makes it an attractive technique to image different types of plaques in patients that suffer from atherosclerotic-related diseases like carotid and peripheral artery disease. One of the current challenges in cardiovascular medicine is predicting which plaques are rupture-prone, and thus have potential to induce myocardial infarction and ischemic strokes. Therefore, VPAT may also play an important role in characterizing vulnerable versus stable plaques, due to its ability to differentiate biological components. Taken together, VPAT has potential to make a significant impact in both research and clinical practice of medicine.
Materials List
| Name | Company | Catalog Number | Comments |
| VPAT Equipment | |||
| Ultrasound System | VisualSonics | Vevo2100 | |
| Nd:YAG OPO Laser | Continuum | Surelite EX | |
| Sapphire Pulse Generator | Quantum Composers | 9200 | 4 ports required |
| BNC Cables | Thor Labs | 2249-C-120 | Outer diameter 0.2’’, length of BNC cable depends on user preference. |
| B connector attached to two BNC cables | L-com | CTL4CAD-1.5 | Continuum also provides this connector |
| Optical Goggles | LaserShields | #37 0914 UV400 | Any goggle with OD 7+ will suffice. |
Transcript
Photoacoustic tomography, PAT, sometimes referred to as optoacoustic tomography, is an emerging biomedical imaging modality that utilizes light-generated acoustic waves to obtain compositional information from a tissue.
Photoacoustic tomography, or PAT, uses particular wavelengths of light to image specific components of the tissue. This is useful for a wide variety of preclinical and clinical applications, such as monitoring lipid-based disease progression.
Currently used imaging techniques are inherently limited in terms of acquisition times, depth of penetration, use of harmful contrasting agents, and costs. PAT, on the other hand, is a rapid, non-invasive, and contrast agent-free technique, which when combined with existing imaging modalities like ultrasound, can provide structural and compositional information simultaneously.
This video will illustrate the basic principles of vibrational PAT and the methodology to set up blood and lipid imaging in mice. Next, we will demonstrate how to interpret VPAT images in conjunction with ultrasound, followed by a few applications of the technique.
Let us begin by discussing the fundamentals of this imaging technique.
During VPAT imaging, single wavelength light from a laser source is shown on the region of interest. This light is then absorbed by a wavelength-specific chemical bond in the biological tissue. In VPAT, the absorbed light causes the molecule to vibrate.
Some of this vibrational energy is then converted to transient heating. This production of heat then causes a thermoelastic expansion of the local tissue and, as a result, produces ultrasonic wave propagation. This is called the photoacoustic effect. The detection of the ultrasonic wave by an ultrasound transducer yields a composition-specific tomographic image.
Mathematically, the light-induced acoustic wave P naught is governed by the temperature-dependent Gruneisen parameter gamma, absorption coefficient mu a, and local optical fluence F. Thus, for each millikelvin rise in temperature, there is an 800-pascal pressure wave that can be detected using an ultrasound transducer. This bond-selective absorption of light allows users to target various biological components by tuning the wavelength of light.
For example, 1,100-nanometer light is used to target blood, and 1,210-nanometer light is used to target lipids. Additionally, since light is being used to induce acoustic wave propagation, this technique can be used to typically image deeper structures than other optical techniques without the need for contrast agents or invasive procedures.
Having reviewed the basics of VPAT, let us now see an example of how to set up and perform VPAT to image blood and lipids in the infrarenal aorta of apolipoprotein E-deficient mice.
First, obtain the necessary equipment: an Nd:YAG pulsed optical parametric oscillator laser, an ultrasound system, a delay generator, and a D connector attached to two BNC cables. Then, attach the Fire BNC cable to port A of the delay generator and Q-switch to port B of the delay generator. Connect the end of the BNC cable from port C to trigger in on the back of the ultrasound system.
Adjust the delay of ports A, B, and C to the values listed here. Ports A and B should specifically output inverted pulses, and port C should output normal pulses. Then, align the fiber optic cable with the laser, and attach the fiber ends to the sides of the 40-megahertz ultrasound transducer.
Now, let’s demonstrate how to prepare an animal for photoacoustic tomography.
First, anesthetize an apolipoprotein E-deficient mouse using 3% isoflurane in a knockdown chamber. Once the animal is anesthetized, move the mouse to the heated stage and secure a nose cone to deliver one to 2% isoflurane. Apply eye lubricant to the animal’s eyes to prevent corneal desiccation. Tape the mouse’s paws to electrodes built into the heated stage to monitor the animal’s respiration and heart rate. Finally, insert a rectal probe to monitor the body temperature.
Next, remove the hair from animal’s entire abdomen by applying depilatory cream. Place the ultrasound transducer on the animal’s abdomen, and locate the infrarenal aorta. The left renal vein and the aortic trifurcation into the tail artery are two landmarks that will help the user locate this area.
To start acquiring images, press B Mode to see a live B Mode image. Adjust the gain using the 2D Gain knob and the focus using the Focal Zone and Focus Depth knobs. Adjust the image width and depth using the Depth Offset, Image Width, and Image Depth buttons.
After this, turn on the laser. Press PA Mode to see live B Mode and PA images. Adjust the PA gain using the 2D Gain knob, and adjust the PA window and color map on the screen. Run the laser at 1,100-nanometer light to target blood, followed by 1,210-nanometer light to target lipids.
Let us now review the results of the VPAT protocol to perform lipid- and blood-specific imaging in vivo.
The ultrasound imaging allowed for obtaining structural information about the infrarenal aorta. This can be used to better interpret the VPAT compositional information. Specifically, the 1,100-nanometer light imaged the blood within the aorta, while the 1,210-nanometer light imaged the subcutaneous and periaortic fat accumulation.
As seen from these images, the subcutaneous fat follows the geometry of the skin. However, the periaortic fat follows the contour of the aorta, and the blood signal originates from within the aorta.
Photoacoustic tomography can be used for a wide variety of preclinical and clinical applications.
In vivo small animal imaging plays an important role in preclinical studies, and photoacoustic tomography uses near-infrared light to detect electronic absorption, enabling the high-resolution imaging of deep brain features for neurobiological applications. Precise data is collected on hemoglobin oxygenation, vascular anatomy, and blood flux. This internal brain imaging information can be used to evaluate normal and pathological brain tissue.
In vascular medicine, it is important to visualize veins and arteries and assess their functionality. Photoacoustic tomography provides compositional information that characterizes plaques as either vulnerable or stable, thus helping to predict which ones are rupture-prone and might induce myocardial infarction or ischemic stroke.
You’ve just watched JoVE’s introduction to photoacoustic tomography. You should now understand the basic principles of this imaging technique and be able to image an animal and interpret the results. Thanks for watching!
