群論の赤外分光法への応用
English
Share
Overview
ソース: タマラ ・ m ・力、化学科テキサス A & M 大学
金属カルボニル錯体は触媒と同様に、有機金属錯体の合成、金属前駆体として使用されます。赤外 (IR) は、CO 含有化合物の最も利用し有益な特性評価手法のひとつです。グループ理論や分子の対称性を記述するための数学を使って、分子内 IR アクティブ C O の振動モードの数を予測する方法を提供します。Ir C O の数を伸ばす実験的観察は、ジオメトリと複雑な金属のカルボニルの構造を確立する直接法です。
このビデオでは、モリブデン カルボニル複雑な Mo(CO)4[P(OPh)3]2シス・トランスフォーム (図 1) であることができるが合成されます。どの異性体は分離を決定するのにグループ理論と IR の分光学を使用します。
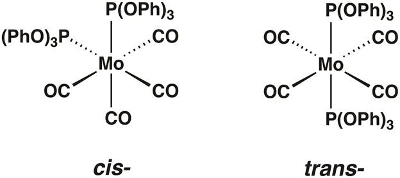
図 1.Cis– とトランス-Mo(CO)4[P(OPh)3]2。
Principles
Procedure
Results
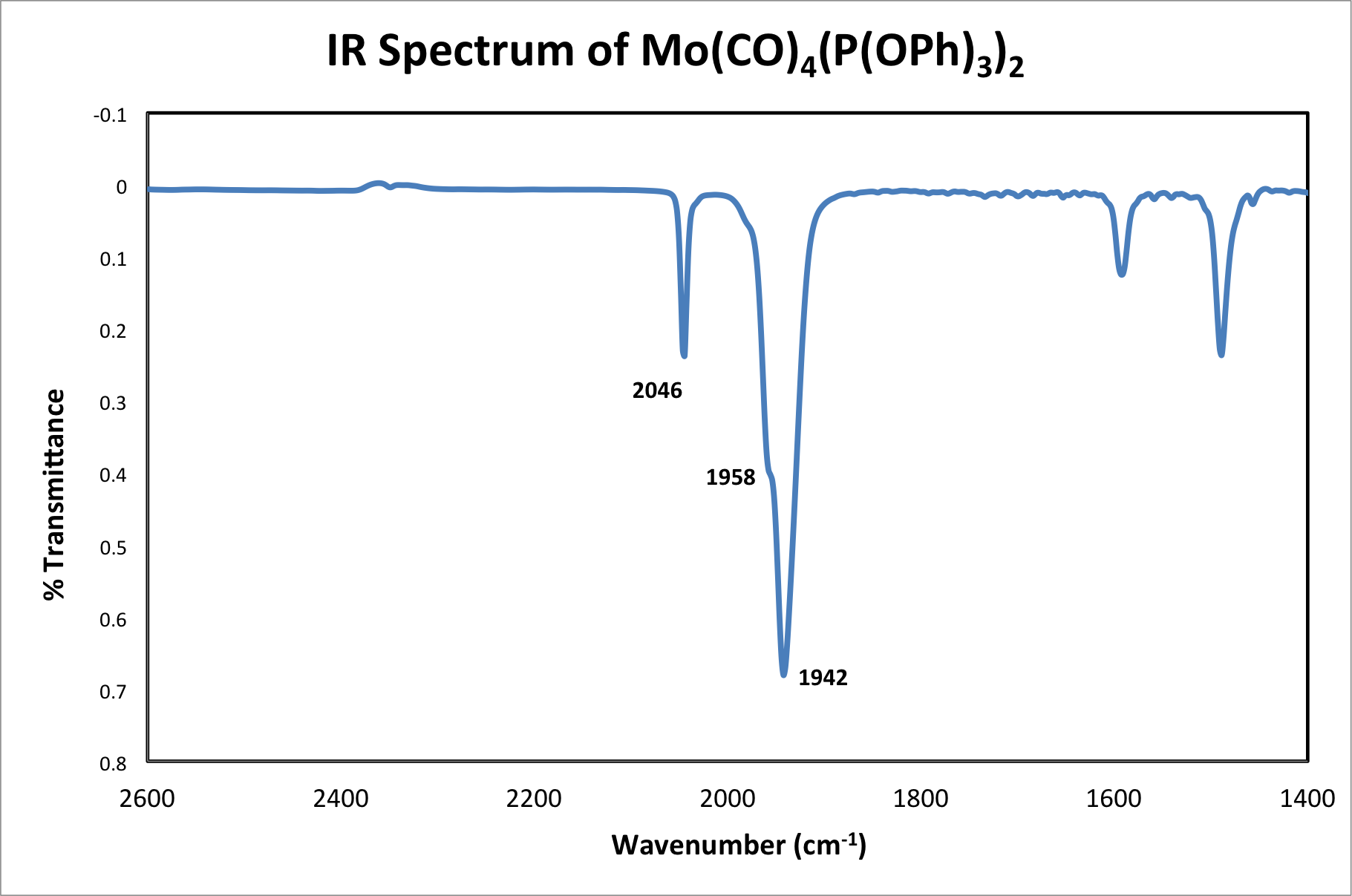
Figure 5. IR of Mo(CO)4[P(OPh)3]2.
Solution IR in saturated hydrocarbon (cm-1): 2046 (s), 1958 (s), 1942 (vs).
The fourth resonance can only be seen under high-resolution conditions. Therefore, it is possible, as in this case, that only 3 of the 4 resonances are observed.
Based on the obtained IR, we can conclude that cis-isomer of Mo(CO)4[P(OPh)3]2 was isolated.
Applications and Summary
In this video, we learned how to use group theory to predict the number of IR active vibrational modes in a molecule. We synthesized the molecule Mo(CO)4[P(OPh)3]2 and used IR to determine which isomer was isolated. We observed that the product had three C-O vibrations in its IR spectrum, which is consistent with the cis-isomer.
Group theory is a powerful tool that is used by chemists to not only predict IR active vibrational modes, but also vibrational, rotational, and other low-frequency modes observed in Raman spectroscopy. Additionally, group theory is implemented in molecular orbital (MO) theory, which is the most widely used model to describe bonding within transition metal complexes. MO diagrams, used by organic and inorganic chemists, can predict and explain a molecule's observed reactivity.
1st, 2nd, and 3rd row metal carbonyl complexes are used widely in inorganic synthesis as metal precursors for more complex organometallic compounds. Some of the most common types of reactions with metal carbonyl complexes include CO ligand substitution, redox at the metal center, and nucleophilic attack at the CO unit. Metal carbonyl complexes themselves are widely used in catalysis. For example, hydroformylation, the industrial production of aldehydes from alkenes, is catalyzed by the metal carbonyl complex HCo(CO)3 (Figure 6).
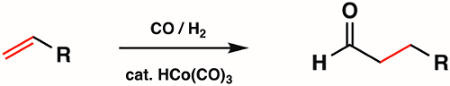
Figure 6. Hydroformylation by the metal carbonyl complex HCo(CO)3.
References
- Fukumoto, K., Nakazawa, H. Geometrical isomerization of fac/mer-Mo(CO)3(phosphite)3 and cis/trans-Mo(CO)4(phosphite)2 catalyzed by Me3SiOSO2CF3. J Organomet Chem. 693(11), 1968-1974 (2008).
- Darensbourg, M. Y., Magdalena, P., Houliston, S. A., Kidwell, K. P., Spencer, D., Chojnacki, S. S., Reibenspies, J. H. Stereochemical nonrigidity in heterobimetallic complexes containing the bent metallocene-thiolate fragment. Inorg Chem. 31(8), 1487-1493 (1992).
- Darensbourg, M. Y., Darensbourg, D. J. Infrared Determination of Stereochemistry in Metal Complexes. J Chem Ed. 47(1), 33-35 (1970).
Transcript
Group theory is a mathematical model connecting molecular symmetry to properties such as IR-active vibrational modes.
Every molecule can be classified with a point group, which describes every symmetry element present in a molecule with respect to a fixed point.
Group theory provides special tables, called character tables, to predict the effect of a molecule’s symmetry on its vibrational modes and other important properties.
This video will discuss the underlying principles of group theory, illustrate the procedure for the synthesis and characterization of an isomer of Mo(CO)4[P(OPh)3]2, and introduce a few applications of group theory in chemistry.
Molecular symmetry describes the indistinguishable configurations of a molecule. The transformations between them are called symmetry operations, which occur with respect to one or more symmetry elements.
The five symmetry elements are proper and improper rotation axes, mirror planes, centers of inversion, and identity. Every molecule has the identity element, or E, in which no change occurs.
A mirror plane, labeled σ, is a reflection plane with identical starting and ending configurations. Molecules may have more than one mirror plane. A center of inversion, labeled i, is a point through which every atom is reflected.
A proper rotation axis is an axis around which a molecule rotates to an identical configuration. It is labeled Cn, where n is 360 divided by the angle of rotation.
An improper rotation axis, labeled Sn, is the axis around which a molecule is rotated and then reflected through a perpendicular mirror plane. Molecules may have more than one rotation axis. The axis with the highest n is the principal axis.
Molecules are assigned to point groups using a symmetry tree, which identifies the symmetry operations needed to classify the molecule.
For example, BF3 is non-linear. It does not have at least two axes with n greater than 2. It has at least one rotation axis; its principal axis is C3. It has three C2 axes perpendicular to its principal axis, and a mirror plane perpendicular to its principal axis. Thus, boron trifluoride belongs to the D3h point group.
Each point group has a character table listing its essential symmetry operations. Each row contains an irreducible representation of the operations, along with the corresponding atomic orbitals and linear movements.
Reducible representations are generated by evaluating how these symmetry operations affect molecular properties. Reducing this representation gives the contributing irreducible representations.
Now that you understand the principles of group theory, let’s go through a procedure for synthesizing an isomer of Mo(CO)4[P(OPh)3]2 and comparing its IR spectrum to the number of IR-active modes predicted for each isomer by group theory.
To begin the procedure, close the Schlenk line vent, and start the flow of N2 gas. Turn on the vacuum pump and with the system at its minimum pressure, cool the vacuum trap with dry ice in acetone.
In a fume hood, measure out 0.5 g of Mo(CO)4(nbd) and place the molybdenum precursor into a 200-mL Schlenk flask. Equip the reaction flask with a stir bar and stopper the flask with a glass stopper. Connect the flask to the Schlenk line via the side arm, and prepare the flask for cannula transfer by evacuating the vessel for 5 minutes, followed by refilling the flask with N2. Repeat this evacuation and refill process a total of 3 times.
Next, prepare another Schlenk flask fitted with a rubber septum containing 20 mL of CH2Cl2. Connect the flask to the Schlenk line and secure the flask in the hood. Using a syringe, draw up 0.87 mL of triphenyl phosphite and dispense it into the Schlenk flask. Make sure the Schlenk line stopcock is open to N2. Degas the CH2Cl2/triphenyl phosphite mixture by bubbling N2 through the solvent for 10 minutes. Then use cannula transfer to add the solution to the flask containing the solid. Open the reaction flask to N2 gas and stir the mixture at room temperature for 4 hours.
Once the reaction has finished, replace the rubber septum with a glass stopper, and remove volatile solvents under vacuum.
Add hexanes to the resulting product and cool in a dry ice/acetone bath briefly, until a precipitate forms. Filter the precipitate and wash the precipitate twice with 10 mL of cold hexanes and collect the solid by filtration. Dry the solid product under vacuum for 15 minutes.
Lastly, dissolve a portion of the product in hexanes and load the solution into an IR cell. Acquire an IR spectrum of the complex.
Now, let’s determine whether the product is the cis or trans isomer by assigning point groups to both isomers and comparing the predicted IR-active modes to the IR spectrum.
Neither the cis nor trans isomer is linear, and neither has more than two rotation axes with orders higher than 2. Both have at least one rotation axis. The principal axes for the cis and trans isomers are C2 and C4, respectively.
The cis isomer does not have two C2 axes perpendicular to its C2 axis, nor does it have a perpendicular mirror plane. It has two mirror planes containing the C2 axis, so its point group is C2v. The trans isomer has four C2 axes and a mirror plane perpendicular to its C4 axis, so its point group is D4h.
Next, reducible representations of the CO stretches are generated by applying each symmetry operation to the molecule and counting the C-O stretches that do not change locations in space.
The C2v table has four operations: identity, C2 rotation, and reflections through two mirror planes containing the C2 axis. In the identity operation, all four dipole moments remain in place. All four dipole moments take different positions after a C2 rotation. Two dipole moments remain in the same position for each reflection.
The reduction formula calculates the coefficient of each irreducible representation in the reducible representation. The point group order is the number of symmetry operations. Classes are types of symmetry operations. Here, the number of operations in each class is 1, which is traditionally omitted from a character table.
The character is the value corresponding to a representation for a given class. When the reduction formula is applied, three irreducible representations are found, with one occurring twice. These representations transform as either the x, y, or z axis, which is consistent with four IR-active C-O stretches.
Using the same technique, the trans isomer is found to have one IR-active C-O stretch. The IR spectrum of the molybdenum product has peaks at 2046, 1958, and 1942 cm-1. With higher resolution data, a fourth C-O stretch may be observed. Based on the obtained IR, one can conclude that the isolated Mo(CO)4[P(OPh)3]2 complex is the cis isomer.
Group theory is widely used in organic and inorganic chemistry. Let’s look at a few examples.
Raman spectroscopy detects molecular vibrations that involve changes in polarizability in the electron cloud. A symmetrical stretch in CO2 does not change the dipole moment, and therefore is not IR-active. However, electrons moving away from nuclei change the polarizability, making the stretch Raman-active. Group theory can identify Raman-active vibrational modes by following the same general method used to identify IR-active modes.
Molecular orbital theory, or MO theory, is a model used to describe bonding in molecules. Adding and subtracting the atomic orbitals of two atoms leads to the formation of molecular orbital diagrams of simple diatomics.
To generate MO diagrams of transition metal complexes, scientists use group theory to generate symmetry-adapted linear combinations of atomic orbitals to represent the outer atoms or ligands. This is achieved by generating reducible representations of the ligand atomic orbitals, and then reducing this to an irreducible representation.
The symmetry representations of the metal center and the symmetry-adapted linear combinations are compared in the diagram. In this model, orbitals with the same symmetry overlap to form two molecular orbitals.
You’ve just watched JoVE’s introduction to group theory. You should now be familiar with the underlying principles of molecular symmetry, finding the point group of a molecule, and some examples of how group theory is used in organic and inorganic chemistry. Thanks for watching!
