히드로겔 합성
English
Share
Overview
출처: 앰버 N. 배런, 애슐레아 패터슨, 테일러 D. 스파크스,재료 과학 및 공학부, 유타 대학교, 솔트레이크시티, UT
하이드로겔은 비교적 간단한 절차와 일반적으로 저렴한 재료로 생산되는 다목적 종류의 교차 연결된 폴리머입니다. 그(것)들은 용액에서 형성될 수 있고 단량제 시약에서 형성된 폴리머 백본, 폴리머 반응성 및 함께 폴리머 사슬을 묶는 교차 연결 종을 만드는 시노레이터를 관련시킵니다. 이러한 물질의 중요한 측면은 물 가에서 팽창한다는 것입니다,하지만이 반응은 염도, pH, 또는 다른 신호의 함수로 붓기를 향상시키기 위해 더 조정할 수 있습니다. 최종 제품으로, 하이드로겔은 수성 또는 건조한 환경에서 사용할 수 있으며 유연성, 높은 흡광도, 투명성 및 단열재와 같은 다양한 유용한 특성을 사용할 수 있습니다. 액체 흡수도, 센서, 소비재 및 약물 전달에 일반적으로 사용됩니다.
Principles
Procedure
Results
The final hydrogel monomer is shown in Figure 7, and the synthesized hydrogels are shown in Figure 8. The degree of swelling was found to be approximately 136% for the 1 min sample, 387% for the 1.5 min sample and 81% for the 5 min sample. These results demonstrate the relationship between degree of crosslinking, or the extent to which the network is connected, and swelling ability. More links between the polymer molecules mean more elastic restraining forces on those polymer chains, which inhibit them from expanding to the same degree as a less-crosslinked hydrogel.
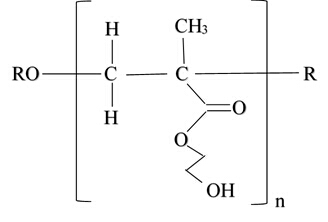
Figure 7: Monomer created from photoinitiator DMPAP, HEMA backbone, TEGDMA crosslinker, EG solvent and photochromic pigment after free radical polymerization.

Figure 8: Hydrogels after polymerization. From left to right: 1 minute under UV light during polymerization, 1.5 minutes under UV light during polymerization, 5 minutes under UV light during polymerization. The 1-minute sample appears more transparent and gel-like then the 1.5 minute and 5 minutes samples, which had increasing degrees of polymerization
Applications and Summary
Hydrogel synthesis is a technique for producing crosslinked polymeric materials which can swell in response to liquid, UV light, pH, or a range of other stimulants. Synthesis by combination of liquid solutions is advantageous for the simplicity of mixing and forming hydrogels, though the final product is generally impure and tends to contain polymers with low molecular weights. This specific procedure, while simple, involves chemicals that are both toxic and flammable, and therefore requires extreme care and preventative measures. The hydrogels produced by this method are useful in applications ranging from drug delivery to sensors to absorbent hygiene products.
Hydrogels are used in a variety of consumer products, medical devices, and sensors. Consumer products such as hospital pads, feminine hygiene pads, and diapers contain sodium polyacrylate, one of the most common superabsorbent polymers. The hydrogel swells in the presence of the fluid between 300-800 times its weight. This allows manufacturers to use less material and create products that are slim and comfortable for the user to wear.
Additionally, soft contact lenses are made of silicone hydrogels, which allow oxygen to easily pass to the cornea and are more comfortable than hard contact lenses. Hydrogels are also commonly used in drug delivery because the cross-linked network allows for drugs to be stored in the three-dimensional network and slowly released into the body.
Hydrogels can also be tuned to swell as a function of salinity, pH, or other signals, making them suitable in sensor applications. The hydrogel synthesized in this video is used as a sensor in a sprinkler lawn sensor. The hydrogel is in contact with the soil and while the lawn is being watered, it swells until it triggers the sprinkler shut-off.
Transcript
Hydrogels are a versatile class of cross-linked polymers, produced through relatively simple procedures using generally inexpensive materials. They are commonly used for liquid absorbents, sensors, consumer products and drug delivery. Hydrogels can be formed from solution, with an initiator making monomerie agents reactive to form a polymer backbone. A crosslinking species then binds the polymer chains together. An important aspect of these materials is that in the presence of water they swell. But this response can be tuned further to enhance swelling as a function of salinity, PH or other signals. Hydrogels can be used in aqueous or dry environments, with a range of useful properties such as flexibility, high absorbance, transparency and thermal insulation. This video will illustrate the synthesis and characterization of hydrogels.
Hydrogels are capable of absorbing hundreds of times their weight in water. When water enters the crosslinked polymer network, it solubilizes hydrophilic, ionic or both species on the polymer backbone. The water molecules are larger than the solubilized groups. Because of this, their presence inside the network causes the hydrogel to swell. While the crosslinks connecting the polymer backbone prevent it from dissolving or breaking. Hydrogel synthesis is a technique for producing these crosslinked, polymeric materials. This is a simple procedure but involves chemicals that are both toxic and flammable and therefore requires extreme care and preventative measures. Using pre-gel constituents, hydrogels can be made via Free Radical Polymerization. One method starts with DMPAP as a Free Radical Initiator.
The carbon-carbon bond in DMPAP is cleaved by ultra-violet light to form an unpaired, highly reactive electron called a free radical on each carbon atom. The free radicals react with the carbon-carbon double-bond in HEMA to form a propogating chain with a free radical on the end. The hydroxal group coming off the backbone is soluble in water, causing the cross-linked network to swell when water is present. The radicals also react with the two carbon-carbon double-bonds in TEGDMA, the chemical crosslinker. This links the backbone chains together. When the free radicals have been consumed, or have completely reacted, the hydrogel synthesis is complete. Swelling can be assessed by drying, hydrating and then re-drying the polymer. In the next section, we will synthesis and characterize hydrogels using this free radical polymerization method.
Prior to beginning hydrogel synthesis, gather the necessary materials and chemicals. The glass slides in the previously assembled synthesis mold are offset by a few millimeters, to create a channel for pipetting the pre-gel solution into the mold. All work should be performed with proper Personal Protective Equipment in a fumehood, as this process involves chemicals that are both toxic and flammable. First, add 0.0012 mol percent DMPAP to the 1000 microliter test tube. Next, use a new pipette each time to add 21.2121 mol percent HEMA and then 3.0303 mol percent TEGDMA to the test tube. Mix the solution using a vortex machine until a homogenous solution is achieved. Dip the spatula into the pigment BCP and rinse it into the solution using 75.7576 mol percent of the solvent ethylene glycol.
Mix the solution using the vortex machine until the pigment completely dissolves and the solution is homogenous. This pigment is used to make the transparent hydrogel visible, while the solvent dissolves the Free Radical Initiator and keeps the hydrogel flexible. Deposit the solution into the mold using a micro pipette aligned with the offset edge of the synthesis mold. Uniformly inject the pre-gel solution into the center of the mold. Place the filled mold five centimeters below a UV-emitting flashlight and irradiate the mold for one minute. Remove the mold from the light and disassemble to remove the hydrogel from the glass slides.
When fully networked, the hydrogel should be a rubber solid with a jello-like consistency. Rinse both sides of the hydrogel with deionized water to remove any unreacted chemical species and polygimers from the product. Repeat this procedure with UV light exposure times of 1.5 and five minutes to produce a total of three hydrogels.
Submerge the finished hydrogels in a container with isopropyl alcohol for one to two hours. The alcohol will replace the ethylene glycol in the hydrogel, allowing it to dry quickly while maintaining its structure. Remove the hydrogels from the alcohol and leave to dry in the open for about 30 minutes. Weigh and record the weight of each of the dried hydrogels. Submerge the hydrogels in deionized water until completely swollen. Remove the gels from the water and gently wipe dry. Weigh and record the weight of the swollen hydrogels. Use the weight of the swollen hydrogels, Ws, and the dried hydrogels, Wd, to calculate the swelling degree.
The degree of swelling was found to be approximately 136% for the one minute sample, 387% for the 1.5 minute sample, and 81% for the five minute sample. These results show that at the longest UV exposure time, there was less swelling. Due to the formation of more links between polymer molecules with increased UV exposure, there were more elastic restraining forces on the polymer chains. This resulted in hydrogels with more crosslinking expanding less than those with less crosslinking.
Now that you appreciate the methods of synthesizing and characterizing hydrogels, let’s take a look at how they are used in everyday products. Consumer products such as hospital pads, feminine hygiene pads and diapers, contain one of the most common super-absorbant polymers. This hydrogel can swell to absorb fluids up to 800 times its weight, allowing manufacturers to create products that are slim and comfortable. The hydrogel synthesized in this video is used as a sensor in a lawn sprinkler. The sensor is in contact with the soil and swells while the lawn is being watered, until it triggers the sprinkler shutoff.
You’ve just watched Jove’s introduction to hydrogel synthesis. You should now understand how hydrogels are synthesized and characterized. Thanks for watching.
