薄膜电镀
English
Share
Overview
资料来源:洛根·基弗、安德鲁·法尔科夫斯基和泰勒·斯帕克斯,犹他大学材料科学与工程系,盐湖城,犹他州
电镀是一种利用电流来减少溶解金属阳离子,使其在电极上形成薄涂层的过程。其他薄膜沉积技术包括化学气相沉积 (CVD)、自旋涂层、浸渍涂层和溅射沉积等。CVD使用要沉积的元素的气相前体。旋转涂层将液体前体向心传播。浸渍涂层类似于旋转涂层,但基板完全浸入其中,而不是旋转液体前体。溅射使用等离子体从目标中去除所需材料,然后对基板板板。CVD 或溅射等技术可生产非常高质量的薄膜,但制作速度很慢且成本很高,因为这些技术通常需要真空大气和小样本尺寸。电镀不依赖于真空大气,这大大降低了成本,提高了可扩展性。此外,电镀可以达到相对较高的沉积率。
Principles
Procedure
Results
Qualitatively, the ITO coated in Prussian Blue, will become transparent when a negative potential is applied as shown in Figure 1 below. This change can be reversed by applying a positive voltage.
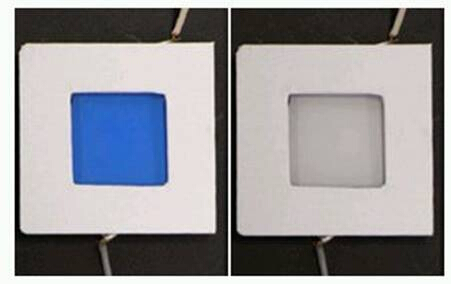
Figure 1: Prussian Blue in its colored and bleached states.
More qualitatively, the thickness of the deposited layer can be changed and measured in various ways including by changing the electrodeposition voltage or the electrodeposition time. For Prussian Blue, varying layer thicknesses will affect the percent transmittance of light through the sample. The relationship between the amount of Prussian Blue deposited on the ITO and the degree of opacity can be measured through UV-Visible spectrophotometry and is shown in Figures 2 and 3.
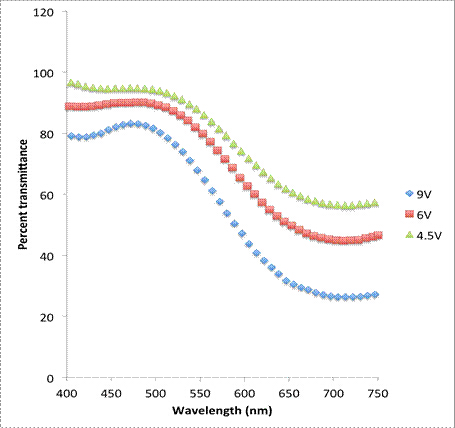
Figure 2: UV-Vis spectroscopy of Prussian Blue in its colored state for various electrochemical deposition voltages.
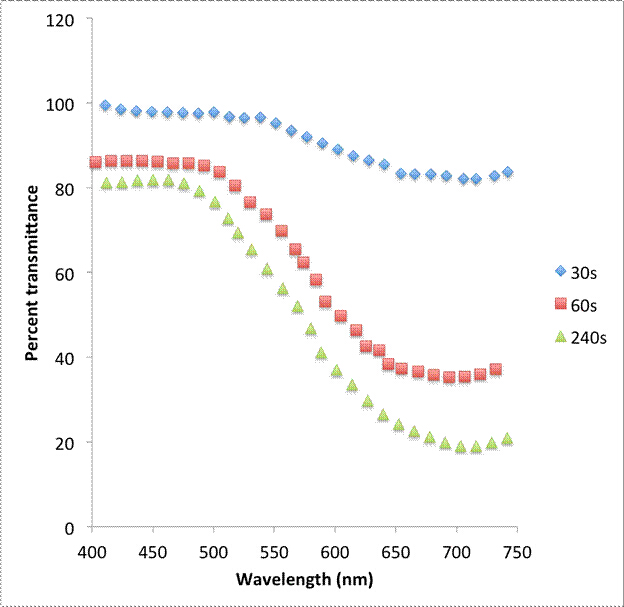
Figure 3: UV-Vis spectroscopy of Prussian Blue in its colored state for various electrochemical deposition times.
Films deposited at a higher voltage saw a lower percent transmittance than those deposited at a lower voltage. This indicates that the layers are thicker at higher voltages than at lower voltages. Additionally, samples electrodeposited for longer times saw lower percent transmittances, again indicating that the films are thicker at longer deposition times.
Applications and Summary
Electrodeposition, as demonstrated in this experiment, allows for the modification of a materials surface properties within minimal change in volume. In the process of electrodeposition, an electric current is passed through an electrolytic solution between an anode and a cathode. The positively charged cations in the electrolyte solution are attracted to and deposited onto the negatively charged cathode. Once deposited, the atoms in the layer gain electrons through the process of reduction.
The speed and amount of electrodeposition depends on the strength of the electric current applied between the cathode and anode in the electrolyte solution. Additionally, the metals used in electrodeposition must be chosen carefully, as some metals will alloy with one another; in those cases, multiple metal layers must be deposited.
Because the cations are chemically bonded the substrate, electrodeposition has the advantages of unified thermal expansion, better resistance to chemical corrosion, and increased physical durability. One drawback of electrochemical deposition compared other methods of thin film deposition is the necessity of a conductive surface on the substrate prior to deposition. Additionally, the process of electrodeposition does not always yield uniform deposition, which causes inconsistencies in the coating of the material.
Electrodeposition has many applications beyond depositing Prussian Blue. Electrodeposition is used extensively in the jewelry industry as it allows for a high degree of control over the plating process and allows for varied aesthetic modifications. A wide variety of color variations can be achieved by depositing different metals to form alloys with unique appearances. Additionally, metals can be deposited in a uniform fashion, which reduces color inconsistencies and can hide solder and component lines. By utilizing electrodeposition, jewelers are able to create functional and consistent metal coatings that are aesthetically pleasing.
Electrodeposition is also used in the automotive industry. Vehicles are constantly subject to forces that wear to vital components. Electrodeposition allows for the properties of various parts to be modified and enhanced without changing functional volume of the part. Deposited chromium provides superior wear and corrosion protection for vehicles and allows cars to last longer with minimum requirements for maintenance and repair.
In the semiconductor industry, electrodeposition offers significant cost, reliability and environmental advantages over classical evaporation technology and can accommodate vastly different wafer sizes. The electrodeposition process allows for deposition on fragile substrates and also permits advanced shape control or new functionalities. Electrodeposition offers a means of inexpensively unique samples by utilizing a technology readily adapted to industrial production.
Transcript
Electroplating is a process that uses electric current to reduce dissolved metal cations onto an electrode surface, forming a thin film. Thin films are a layer of material that range in thickness from less than one nanometer to several micrometers. These thin films are used in a wide range of applications, ranging from solar cells to biosensor probes, and provide modified surface properties with minimal change in volume. However, it is essential that the thickness of the thin film is consistent and controllable. There are many different thin film deposition techniques commonly used to controllably deposit thin films, and each has its own benefits and drawbacks. In this video, we will introduce the electroplating technique, and demonstrate how to form a thin film using this method in the laboratory.
Electroplating is performed in a set up like a galvanic cell which consists of two different metals, an anode and a cathode, connected by a salt bridge or porous membrane. These electrochemical cells have oxidation and reduction half-cell reactions that spontaneously occur at each of the metal electrodes, thereby generating electrical current. Electroplating relies on a similar concept. However, it reverses it by supplying current, thereby driving non-spontaneous redox reactions. The anode is made of the metal to be plated and is oxidized, creating dissolved ions. These ions flow through the electrolytic solution, which contains metal salts and other ions which permit the flow of electricity.
The dissolved metal ions are then reduced and plated on the cathode. The electroplating process requires that both the anode and cathode materials are conductive. Thus, metals are typically used. Plating thickness is controlled by varying the duration and strength of the electric current between the electrodes. Increasing either or both of these parameters will result in thicker plating layers. Now that you’ve learned the basics of electroplating, we will demonstrate the technique by plating a thin film of the dark pigment, Prussian blue, onto a sheet of polyester coded with indium tin oxide, or ITO.
To begin, prepare the Prussian blue solution. Prussian blue is a pigment produced by the oxidation of ferrocyanide salts. Mix 50 milliliters of 0.05 molar hydrochloric acid, 100 milliliters of 0.05 molar potassium hexacyanoferrate (III), and 100 milliliters of 0.05 molar iron (III) chloride hexahydrate. Now, create an anode by wrapping about eight centimeters of Nichrome wire into a tight coil. Prepare the cathode by first cutting the ITO-coated polyester into a five by five centimeter square. Then remove the outer coating that protects the conductive side of the material.
Next build the circuit by connecting the positive terminal of a nine volt battery in series with a 30 kiloohm resistor. Then connect it to the Nichrome anode using an alligator clip. Connect the negative end of the battery to the ITO cathode using an alligator clip. Make sure that the anode and cathode are not touching. Now, lower the cathode and anode into the Prussian blue solution, taking care not to submerge the alligator clips. Hold the setup in the solution for one minute. Then remove and rinse the two electrodes in deionized water. Repeat the process with new ITO electrodes, each submerged for different deposition times and battery voltages.
Now we’ll analyze the various films using percent transmission of visible light in the range of 750 to 400 nanometers via UV-VIS spectroscopy. First, perform a background scan using a ITO substrate that has not been coated with Prussian blue. Then, measure the percent-transmission of the Prussian blue coated samples, subtracting the background transmittance from the blank ITO. Now compare the percent transmittance between each of the samples. First, let’s take a look at the effective deposition time. These samples were deposited for 30, 60, and 240 seconds. The percent transmittance was lower for samples with longer deposition times, indicating thicker films. Similarly, films deposited at higher voltages showed lower transmittance than those deposited at lower voltages, indicating the formation of thicker films at higher voltages.
Thin films had a wide range of applications in materials engineering and other fields of research. The electroplating technique can be used to pattern micro-scale features and nano-scale thickness onto a surface. Here, researchers spin coated photoresist onto a conductive substrate. And then patterned a microscale lattice using a mesh-patterned mask using UV light. The UV-exposed patterned was then removed using developer solution to reveal a lattice pattern of trenches that reveal the conductive substrate. Copper was then electroplated onto the surface with the metal film forming only on the conductive parts of the substrate and not on the remaining photoresist.
After removal of the remaining photoresist pattern, a lattice of raised metal remained, with a thickness of less than two nanometers. Electroplating can also be used to deposit layers of biological materials on a surface, thereby improving the bio-compatibility of a sensor or probe. Here, a thin film of chitosan was electro deposited onto a patterned gold cathode. Chitosan, a polysaccharide, is soluble below pH 6.3 and insoluble above pH 6.3. Water electrolysis at the cathode induced a local increase in pH, which caused the sol-gel transition of the material, making the deposited film insoluble. This enabled its use as a bio-compatible surface for enzyme adhesion and the development of a glucose sensor.
You have just watched JoVE’s introduction to the electroplating of thin films. You should now understand how the electroplating process works, how it is performed in the lab, as well as some applications of this technology. Thanks for watching.
