Degasaggio di liquidi con ciclo freeze-pump-thaw
English
Share
Overview
Fonte: Laboratorio del Dr. Neil Branda — Simon Fraser University
Il degasaggio si riferisce al processo mediante il quale i gas disciolti vengono rimossi da un liquido. La presenza di gas disciolti come l’ossigeno o l’anidride carbonica può impedire reazioni chimiche che utilizzano reagenti sensibili, interferire con le misurazioni spettroscopiche o indurre la formazione di bolle indesiderate.
Sono disponibili diverse tecniche per il degasaggio dei liquidi; alcuni di questi includono riscaldamento, agitazione ultrasonica, rimozione chimica dei gas, sostituzione con gas inerte mediante gorgogliamento e ciclo congelamento-pompa-scongelamento. Il ciclo di congelamento-pompa-disgelo è un metodo comune ed efficace per il degasaggio su piccola scala e sarà dimostrato qui in modo più dettagliato.
Principles
Procedure
Applications and Summary
The removal of dissolved gases is important in both academia and industry. It is often required for maintaining the quality of machinery and laboratory instruments, for protecting various chemical reactions, and obtaining accurate readings for chromatography and spectrophotometry.
Reactions that use or generate air sensitive reagents, for example, organometallic compounds, thiols, phosphines, and electron rich aromatics frequently require some level of degassing to maintain their integrity throughout the experiment. The yield or even the outcome of an air sensitive reaction could be altered if the appropriate precautions to remove dissolved gases are not taken. Dissolved oxygen affects photochemical studies by quenching excited states. For instance, aromatic triplet states can be quenched by small quantities of oxygen present in the solution, affecting the intensity and spectral distribution (Figure 3).
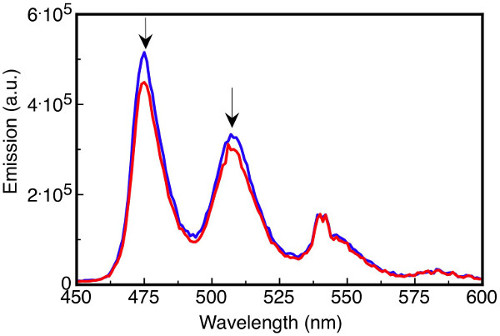
Figure 3. Fluorescence emission spectra of the solutions. Tetracene (16 µM) in degassed benzene (blue line) and oxygen-saturated benzene (red line) upon excitation at 410 nm where the emission intensity at 475 nm is reduced by 14% in the oxygen-saturated solution.
In industry, water is a commonly used fluid for heat exchange. The lifetime of metal pipes, boiler systems, and pumps is dependent on the quality of the running water. Water contains different levels of oxygen and carbon dioxide, can cause damage to metallic materials. Oxygen is an oxidizing reagent, and carbon dioxide is corrosive due to its conversion to carboxylic acid. Delivery of degassed water to the above mentioned systems will prolong equipment lifetime.
In addition, gases present in solvents can have negative consequences for laboratory instruments such as in high-performance liquid chromatography (HPLC) with respect to both performance and output. Many of the instruments have metal propellers or pumps that distribute solvent. When in contact with solvent that has dissolved gas, it can cause cavitation and corrosion leading to damage or degradation of metal components. The detector stability is also influenced by the presence of dissolved gases and the insufficient removal of oxygen can cause baseline drift.
Freeze-pump-thaw cycling is a relatively quick and efficient method appropriate for small to medium scale degassing of liquids. This process can help to overcome some of the issues discussed above associated with the presence dissolved gases in the solvent.
References
- Shriver, D. F., Drezdn, M. A. The Manipulation of Air Sensitive Compounds. 2nd ed. Wiley & Sons: New York, NY (1986).
- Girolami, G. S., Rauchfuss, T. B., Angelici, R. B. Synthesis and Technique in Inorganic Chemistry: A Laboratory Manual. 3rd ed. University Science Books: Sausalito, CA, (1999).
- Kotz, J., Treichel, P., Townsend, J. Chemistry and Chemical Reactivity. 8th ed. Brooks/Cole: Belmont, CA. (2012).
Transcript
The degassing of liquids is imperative to many chemical synthesis techniques in organic chemistry. Degassing refers to the process by which dissolved gases are removed from a liquid. Degassing is important in cases where chemical species are susceptible to unwanted reactions with oxygen. Freeze-pump-thaw cycling is a common method utilized for the small scale degassing of liquids. The technique is performed under reduced pressure using a Schlenk line, or vacuum/inert gas double manifold. This video will outline the principles of performing freeze-pump-thaw degassing in the laboratory.
Freeze-pump-thaw degassing takes advantage of the pressure dependence of the gas’s solubility in a liquid. This is why soda bubbles when opened, indicative of Henry’s law. According to Henry’s Law, the mole fraction of a gas dissolved in a liquid is directly proportional to the partial pressure of the gas in the vapor phase above the liquid. Thus, by lowering the pressure of the gas above the liquid, the solubility of the dissolved gas decreases, and is then released as bubbles.
Freeze-pump-thaw degassing involves first freezing the solvent using a Dewar of liquid nitrogen or dry ice. A vacuum is then applied, and the headspace above the frozen solvent evacuated. This decreases the pressure in the headspace above the liquid, thereby lowering the solubility of the dissolved gas.
The flask is then sealed and the solvent is thawed, enabling the release of dissolved gaseous species into the headspace. The liquid is then refrozen, and the process repeated as many times as necessary.
Freeze-pump-thaw degassing is typically performed with a Schlenk line setup, as it involves the application of a vacuum, as well as the introduction of inert gas. A Schlenk line consists of a dual glass manifold with multiple ports. This collection’s video on the Schlenk line will go into more detail about this apparatus. Now that the basics of the freeze-pump-thaw technique have been described, the procedure will be demonstrated in the laboratory.
First, obtain a clean, dry Schlenk flask. Inspect the flask for cracks or fractures, which may cause the flask to shatter during the process.
Secure the Schlenk flask with a clamp, and add the desired solvent or solution. Do not use more than 50% of the volume, as some solvents expand upon freezing, which could shatter the flask. Close the stopcock, and ensure that any openings are sealed. Connect the side arm of the Schlenk flask to the Schlenk line with a piece of flexible tubing, and keep the corresponding valve on the Schlenk line closed. Open the stopcock on the flask, as well as the valve connected to the vacuum line to evacuate the flask. Once vacuum is established, close the valve. Open the valve to the inert gas line to fill the flask. Once filled with inert gas, close the stopcocks on the flask and then on the line.
Submerge the flask into a Dewar containing liquid nitrogen in order to freeze the liquid. When the solvent is frozen, open the stopcock on the Schlenk flask, and the valve on the Schlenk line to pull a vacuum in the flask. Keep the flask under vacuum and inside the liquid nitrogen Dewar for about 10 min.
Remove the flask from the liquid nitrogen Dewar. Next, seal by closing the stopcock.
Immerse the flask in a warm water bath in order to fully melt the solvent. During this procedure, gas bubbles will visibly evolve from the solvent. Do not disturb the liquid, and allow the solvent to thaw by itself.
Once the solvent has thawed completely, replace the warm water bath with the liquid nitrogen Dewar, and refreeze the solvent.
When the solvent is frozen, open the stopcock on the Schlenk flask and on the Schlenk line to pull a vacuum in the flask. After 10 min, close the stopcock on the flask and Schlenk line, then remove the liquid nitrogen Dewar. Thaw the solution again in a warm water bath. Repeat the process until gas bubbles no longer evolve from the solvent.
After the completion of these cycles, seal the Schlenk flask under inert gas. To do so, open the valve to the inert gas on the Schlenk line, and then open the stopcock of the flask to expose the solvent to a inert atmosphere.
When the Schlenk flask is filled with gas, close the Schlenk flask and Schlenk line valves. The solution is now degassed and ready to use.
Degassing techniques are vitally important for applications where the presence of certain gases is either hazardous, or may contaminate an experiment.
Degassing of solutions for organic synthesis is a key application of a Schlenk line system. In this experiment, cadmium selenide nanocrystals were synthesized, where oxygen is detrimental to the reaction. First, molecular precursors were prepared and heated. The mixture was degassed under vacuum, and then the flask flushed with argon. The reaction was then completed under argon atmosphere.
The Miller-Urey experiment is a pioneering study focused on the origins of life. The experiment requires that only gases in a primordial atmosphere are present. First, the primordial atmosphere was recreated in a sealed round bottom flask containing water to simulate the oceans. It was fitted with electrodes that simulate lightning. The liquid was degassed using a Schlenk line, prior to introducing primordial gases such as ammonia and methane.
The closed flask containing the gases was removed from the system. Sparking was then conducted to simulate lightning in the primordial soup. A number of amino acids and other small organic molecules were generated.
Degassing can also be conducted using a vacuum chamber in cases where ambient air will not contaminate the solution. In this example, polydimethylsiloxane pillars were molded from a previously prepared mold. The molded apparatuses, known as microfluidic devices, are used to finely control small volumes of liquid. To do this, a 10:1 mass ratio of PDMS base and curing agent were vigorously mixed. The solution was then degassed in a vacuum chamber to remove all bubbles. The degassed polymer was then poured over the mold, and cured in an oven. The devices were then separated from the mold, and used to study surface tension properties of liquids.
You’ve just watched JoVE’s introduction to the degassing of solvents using the freeze-pump-thaw technique. You should now have a better understanding of how to use this technique in a Schlenk line system.
Thanks for watching!
