Transfert de solvants via une rampe à vide (ligne Schlenk)
English
Share
Overview
Source : Hsin-Chun Chiu et Tyler J. Morin, laboratoire du Dr Ian Tonks — Université du Minnesota Twin Cities
Lignes de Schlenk et haute sous vide sont tous deux utilisés pour exclure de l’humidité et l’oxygène de réactions en exécutant des réactions sous une légère surpression de gaz inerte (généralement N2 ou Ar) ou sous vide. Transfert sous vide a été conçu comme une méthode distincte des solvants (autres réactifs instables) d’agents de séchage (ou d’autres agents non volatiles) et les distribuer à réaction ou stockage bateaux tout en maintenant un environnement sans air. Distillations semblables au thermique, transfert sous vide sépare les solvants par vaporisation et de condensation eux dans un autre récipient récepteur ; Toutefois, les transferts sous vide utilisent la basse pression dans les collecteurs de Schlenk et lignes vide élevés à bas point d’ébullition à la température ambiante ou inférieure, permettant la distillation cryogénique. Cette technique peut fournir une alternative plus sûre à la distillation thermique pour la collecte des solvants exempt d’air et d’humidité. Après le transfert sous vide, la teneur en eau du solvant recueillie peut être testée quantitativement par titrage Karl Fischer, qualitativement par titrage avec une solution de2CO Na/Ph ou par 1H RMN.
Principles
Procedure
Results
The picture was taken of the vacuum transfer in progress (Figure 2) and after the Na/Ph2CO titration titration was performed (Figure 3).
Solvents collected via this method have been tested by ketyl titration. Figure 3 shows the common possible outcomes of the ketyl test. The purple color in (a) indicates < 10 ppm H2O in the solvent; while the blue and colorless solutions indicate a wetter solvent that needs further purification prior to use with water sensitive applications.

Figure 1. Glassware needed to make a ketyl pot and perform a vacuum transfer to a Straus flask. (a) Funnel for adding solvent to ketyl pot; (b) 500-mL round-bottom flask; (c) 180° adapter; (d) 500-mL Straus flask; (e) vacuum transfer bridge.

Figure 2. Setup of vacuum transfer: (a) the high vacuum line, (b) the transfer bridge, (c) the solvent pot with 180° adapter, (d) the receiving Straus flask, and (e) cooling bath.
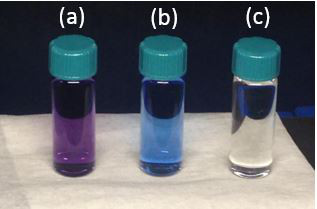
Figure 3. Collected solvent after titration of the ketyl solution. (a) purple indicates < 10 ppm H2O, while (b) blue and (c) colorless require further purification.
References
- Shriver, D. F., Drezdn, M. A. The Manipulation of Air Sensitive Compounds. 2nd ed.; Wiley & Sons: New York, (1986).
- Girolami, G. S., Rauchfuss, T. B., & Angelici, R. B. Synthesis and Technique in Inorganic Chemistry: A Laboratory Manual. 3rd ed.; University Science Books: Sausalito, CA, (1999).
- Szafran, Z., Pike, R. M., & Singh, M. M. Microscale Inorganic Chemistry. Wiley & Sons: New York, (1991).
- Tidwell, T. T. Angew. Chem. Int. Ed. 40 331-337. (2001).
- Chandra, T. University of Wisconsin Madison Chemistry. Safe Chemical Manipulations Using a Schlenk Line. https://www.chem.wisc.edu/content/schlenk-line-techniques (2015).
- Toreki, R. Interactive Learning Paradigms Incorporated. The Glassware Gallery: Schlenk Lines and Vacuum Lines. http://www.ilpi.com/inorganic/glassware/vacline.html (2015).
- Ogryzlo, E. A. Why Liquid Oxygen is Blue. J. Chem. Educ. 42, 647-648 (1965).
- Armarego, W. L. F., Perrin, D. D. Purification of Laboratory Chemicals. 4th ed.; Butterworth-Heinemann: Woburn, (1997).
- Williams, D. B. G., Lawton, M. Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants. J. Org. Chem. 75 8351-8354 (2010).
- Schwartz, A.M. Chem. Eng. News. 56 (24), 88. (1978).
Transcript
Certain chemical reactions must be kept free of water and oxygen. A Schlenk line is a dual manifold used in the safe handling of air- and moisture-sensitive reagents.
The apparatus was invented in the 1920s by Wilhelm Schlenk. A key component of his design is the Schlenk flask. It has a stopcock, where vacuum or inert gas can be applied to the system, as needed. The neck opening can be interfaced with another apparatus, or sealed with a septum, and reagents can be added without the introduction of air.
Once the reagents have been introduced to the apparatus, they can be manipulated in an oxygen- and water-free environment.
This video will highlight the basic operation procedures for a Schlenk line, and then demonstrate the principle in the laboratory via the vacuum transfer of solvents.
A Schlenk line is a tubular glass apparatus, with one line used to deliver a vacuum, and another line used to deliver inert gas. Together, the system is called a dual manifold. The dual manifold has four to six valved ports, with thick rubber tubing leading to various reaction apparatuses. The inert gas manifold is connected to a pressure regulated inert gas source. It is vented through an oil bubbler to keep the line pressure slightly above atmospheric. The oil bubbler also prevents ambient air from entering the manifold, preventing contamination of the line.
The vacuum manifold is connected to a vacuum pump. A cryogenic trap, often cooled with liquid nitrogen or a dry-ice slurry, is located between the vacuum manifold and the pump in order to condense volatile components, thus preventing them from entering and damaging the vacuum pump.
A Schlenk line system can be used for many techniques and reactions, such as the vacuum transfer of solvents. This involves the transfer of solvents from vessel to vessel, while maintaining an air free environment.
Now that you understand the principles of Schlenk line operation, lets see a transfer of air- and oxygen-free solvent.
To begin, make sure that all working ports on the manifold are closed, and that all joints are properly coated with high vacuum grease.
Attach the solvent traps to the vacuum line, and seal them by turning on the vacuum pump.
Place vacuum-sealed Dewars around the solvent trap, and fill with liquid nitrogen to cryogenically protect the pump.
Turn on the regulated inert gas flow, and adjust by watching the bubbler. Connect the desired apparatus, such as a Schlenk flask, to the manifold port using thick rubber tubing or standard taper glassware.
Clear the headspace in the flask by first opening the reaction port to vacuum and fully evacuating the flask. Close the port to vacuum, and then slowly open the reaction port to inert gas and wait until the bubbler begins to bubble again. Close the reaction port to inert gas, and repeat the process two more times.
The next step is to prepare a solvent pot. This is used to produce water- and oxygen-free solvent for sensitive reactions. This procedure uses the common benzophenone-sodium setup.
To begin, place the glassware and reagents into a glovebox under inert atmosphere. Measure approximately one cubic centimeter of sodium metal, and cut it into smaller pieces. Place the pieces into a 500-mL round-bottomed flask with a standard taper neck joint. Weigh 1.25 g of benzophenone and place it into the round-bottomed flask with the sodium. Add a heavy-duty stir-bar.
Seal the flask using a 180° 24/40 adaptor that has been greased with a minimal amount of heavy-duty high vacuum grease. Place a Keck clip over the joint to ensure a secure connection.
Remove the flask from the glovebox, and evacuate the flask headspace using the Schlenk line. Seal the 180° adaptor, and remove the flask from the line while under vacuum.
Attach a funnel to the top of the solvent pot and fill the funnel with roughly 300 mL of the desired solvent. Using a long needle attached to a nitrogen line, bubble nitrogen through the solvent to partially degas it.
While maintaining the nitrogen bubbling, slowly open the 180° adaptor to introduce solvent into the solvent pot. When the solvent level in the funnel approaches the adaptor, close the adaptor and remove the funnel.
Stir the pot for several hours. The solution will turn deep purple indicating the formation of the sodium benzophenone ketyl radical. The formation of the radical signifies that the solvent is dry and oxygen-free. If the pot does not turn deep purple, degas the solution. Use the freeze-pump-thaw technique, as described in detail in this collection’s “Degassing Liquids with Freeze-Pump-Thaw Cycling.”
Dry a 500 mL receiving Straus flask and a U-shaped vacuum transfer bridge in a drying oven. A Straus flask is a two-neck round bottom flask. One neck is threaded to allow for the connection of a plug valve.
Coat all joints lightly with vacuum grease, and attach the U-shaped bridge to the vacuum line. Connect the Straus flask and solvent pot to the U-shaped bridge. Be sure to secure the heavy system in place using Keck clips. Evacuate the system and degas the solvent as described earlier.
Close the top U-bridge valve to close the vacuum. The system should be under static vacuum with the Straus valve open and the solvent pot 180° adapter closed. Use a lab jack to raise a -78 degree acetone/dry ice slurry to cool the receiving Straus flask.
Begin stirring the solvent pot, and then slowly open the stopcock of the 180° adaptor. Be sure to turn the stopcock slowly so the liquid will not rapidly boil into the U joint. Solvent will begin condensing in the receiving flask. If the solvent pot freezes during transfer, close the Straus flask valve and allow the solvent pot to warm to room temperature before continuing.
If solvent transfer is extremely slow, degas the system again using freeze-pump-thaw.
Wait until the solvent pot is almost dry, or until the desired amount of solvent has been collected. Close the stopcock on the receiving flask, and on the solvent pot. The sealed flask can now be removed from the system.
To shut down the system, first close off all manifold ports, and turn off the inert gas flow.
Next, remove the solvent trap and the Dewars. Take extreme caution if any blue liquid is present in the trap, as it could possibly be liquid oxygen. Consult safety protocols for appropriate action.
Schlenk line systems are used in a wide range of air sensitive reactions in organic chemistry.
Quantum dots are widely used for single-molecule fluorescence imaging. In this example, quantum dots were synthesized under an inert atmosphere using a Schlenk line. Small cadmium selenide quantum dot cores were first synthesized under inert gas and vacuum conditions. Their fluorescent properties are dictated by the size of the nanoparticle.
The selenium was swiftly injected into the cadmium solution to complete the synthesis of the cores. They were then functionalized with mercury and biocompatible polymer coatings, increasing their fluorescent yield. The fluorescence duration of the dots far exceeded that of traditional dyes or proteins.
The handling and analysis of volatile and air sensitive gases is typically challenging, but can be safely executed with the use of a Schlenk line. In this example, volatile gases were transferred to a lockable test tube, using a Schlenk line.
The test tube was cooled using liquid nitrogen, in order to condense the gases and trap them in the test tube. The contained gases were then transferred to a mass spectrometer using the locked test tube, and a custom connection system.
You’ve just watched JoVE’s introduction to the Schlenk line system. You should now understand how to operate a Schlenk line, dry and purify solvents, and conduct a vacuum transfer.
Thanks for watching!
