Two-Photon Microscopy for the Study of Tendons
Summary
This article outlines the process of preparing, setting up, and imaging tendons using multiphoton microscopy. Additionally, it covers the application of SHG for analyzing collagen fibril alignment and the creation of a 3D representation of tendons. This methodology proves highly valuable in characterizing tendon cells and their ECM during injury and development.
Abstract
Two-photon microscopy has emerged as a potent tool for evaluating deep tissue cells and characterizing the alignment of the extracellular matrix (ECM) in various biological systems. This technique relies on nonlinear light-matter interactions to detect two distinct signals: the second harmonic generated (SHG) diffusion signal, which facilitates the visualization of collagen fibers and their orientation, and the near-infrared excitation signal for imaging ultraviolet excited autofluorescence.
SHG imaging proves especially effective in visualizing collagen fibers due to the non-centrosymmetric crystalline structure of fibrillar collagen I. Given that tendons are matrix-rich tissues with a limited number of cells, their high collagen content makes them ideal candidates for analysis using two-photon microscopy. Consequently, two-photon microscopy offers a valuable means to analyze and characterize collagen abnormalities in tendons. Its application extends to studying tendon development, injuries, healing, and aging, enabling the comprehensive characterization of tendon cells and their interactions with the ECM under various conditions using two-photon microscopy tools. This protocol outlines the use of two-photon microscopy in tendon biology and presents an adapted methodology to achieve effective imaging and characterization of tendon cells during development and after injury. The method allows the utilization of thin microscopic sections to create a comprehensive image of the ECM within tendons and the cells that interact with this matrix. Most notably, the article showcases a technique to generate 3D images using two-photon microscopy in animal models.
Introduction
To properly function and transmit force from muscle to bone1, tendons rely on the intermolecular and intramolecular bonds between collagen fibers. The intricate self-assembly, crosslinking, and alignment of the collagen fibers result in the establishment of a highly organized matrix that contributes to the biomechanical strength and flexibility of tendon tissue2,3,4. Although other ECM proteins also contribute to the stability of the fibrillar network in tendons5, the tendon dry mass is approximately 86% collagen6, with collagen I making up to 96% of the total collagen content7,8. This ultimately makes collagen structure a key output of normal tendon health and function.
Some clinical imaging modalities used for tendons are MRI and/or ultrasound. While ultrasound technology provides images of the fascicular structure of the tendon and reveals some of the fibrillar structure in the tissue9, the resolution is not ideal for quantification. MRI has better spatial resolution than ultrasound imaging, but it is still limited. However, these methods are also limited in their potential imaging depth through a tissue. On a more microscale, small- and wide-angle light scattering and confocal microscopy can be used to assess the structure of collagen fibrils and any potential abnormalities.
In contrast, second-harmonic generation (SHG) microscopy differs from other imaging methods as it can capture the outer shell of the collagen fibrils. Collagen I fibrils in tendons are organized in a uniaxial parallel manner through the tendon ECM and are non-centrosymmetric in nature4,10. These properties can be leveraged for imaging using a multiphoton microscope, which can produce clear images that are up to 2-3 times deeper than confocal imaging11. This also allows us to generate better quality optical sections of the tendon. When light is projected into the sample of interest, an SHG signal is produced, and this scattering of light can be captured. In tendons, this produces an image of the collagen structure and alignment, thus allowing us to evaluate potential morphological and pathological consequences of tendinopathies, injuries, etc.
Since collagen makes up most of the tendon dry mass and contributes to tendon function6,12, disruption to collagen structure can affect the biomechanical properties of tendons. Thus, analyzing its structure can help us better understand the impact and severity of injuries, as well as create a metric for healing efficacy. This paper reviews a method that uses multiphoton and SHG microscopy to analyze how development and injuries affect the structure and alignment of collagen I fibrils and to generate 3D images of tendon ECM in animal models. Therefore, our method to image tendons may help researchers characterize tendon cells and ECM during development or after injury.
Protocol
All animal experiments were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) (Protocol #2013N0000062) and AAALAC guidelines at Massachusetts General Hospital. BHLHE40 null knockout or heterozygous female mice in a Scx-GFP background, age 30 days, were used for the present study. The animals were obtained from a commercial source (see Table of Materials).
1. Tissue preparation and fixation
- To prepare the tissue, euthanize mice in a CO2 chamber (following institutionally approved protocols) followed by cervical dislocation.
- To help reduce the amount of fur that may stick to the tissue sample, spray the mouse with 70% EtOH until the fur is saturated. Skin the hindlimbs, and then using scissors, remove the two hindlimbs by cutting at the hip joint.
- Place the two hindlimbs in a 20 mL scintillation vial, and immerse them in 4% PFA such that the vial is filled. Place the vials at 4 °C with agitation overnight.
- Wash the hindlimbs in 1x PBS at room temperature (RT) with agitation for 10 min and repeat a total of 3 times. Immerse the hindlimbs in 0.5 M EDTA (pH 8.0) at 4 °C with agitation for up to 2 weeks, replacing with fresh EDTA every 2-3 days.
- Wash the hindlimbs in 1x PBS at RT with agitation for 10 min, repeating the washes a total of 3 times.
- Immerse the limbs in fresh 1x PBS and store at 4 °C until they are used for imaging.
2. Dissection of tendon and preparing tissue for imaging
- While holding the foot with forceps, slide one end of the spring scissors' blade (see Table of Materials) underneath the Achilles tendon and ensure to cut as close to the myotendinous junction as possible.
- Hold the severed myotendinous end of the tendon with forceps and use the spring scissors to cut out the tendon as close to the bone as possible.
NOTE: If needed, use forceps to help hold the Achilles tendon away from the rest of the hindlimb during the first cut. Ensure not to crush or damage the tissue, as it will affect the collagen alignment during imaging. - To permeabilize and have a nuclear counterstain, immerse the dissected tendon in PBT (1% Triton-X in PBS) with 0.1% Draq5 (stock concentration, 5 mM, see Table of Materials). Place the tendon in a 2 mL microcentrifuge tube, and add 1 uL of Draq5 to 1 mL of PBT. Incubate overnight at RT on a rocker. Since Draq5 is light-sensitive, cover microcentrifuge tubes with aluminum foil and protect the samples from light.
- Before imaging, remove the Draq5 solution and replace it with dPBS. Ensure to continue to protect the sample from light.
CAUTION: Draq5 has category-three toxicity and is considered hazardous by the OSHA Hazard Communication Standard. Avoid contact and dispose of it in a labeled waste container. - Using a biopsy punch tool with an inner diameter of 6 mm, cut out a piece of gel foam (see Table of Materials) and place it in a small 60 x 15 mm Petri dish. Add ddH2O to the dish to allow the gel foam to expand.
- Place dissected Achilles tendon on gel foam. Using a coverslip glued to a washer, carefully place the washer over the tendon until the coverslip makes contact with the tissue. To avoid issues when imaging, ensure to have no air bubbles between the sample and the coverslip. If needed, add more ddH2O to the dish to cover the sample and the washer with the coverslip.
3. Imaging with a multiphoton microscope
- Using a FVMPE-RS multiphoton laser microscope, 25x objective that can be immersed in water, HPDS-O IR pulsed laser, and IR pulsed laser (see Table of Materials), locate the sample and region of interest that will be imaged using the EPI light path. Once the sample is located, set the camera on either the myotendinous or entheseal end of the Achilles tendon.
- Switch the light path to LSH, adjust Laser 2's IR Laser setting to 840 nm, and actively align. Adjust the HV strength to be able to see the tissue and the SHG signal, without exceeding 20%. Do not adjust the gain and keep the offset equal to 0.
- Then, configure the z-stack parameters to acquire the sample images. Use a 1024 x 1024 scan size and set the stepsize to 0.4 microns. Z-stacks are, on average around 150 slices, and they start from the outside sheath and move toward the center of the tendon until the SHG signal is no longer clear, producing sagittal optical sections13.
- Adjust the laser power to Bright Z mode to allow for signal detection standardization. Once the Z-stack start/stop parameters are established, set the laser intensity from 4 at the start of imaging and 6 at the end of imaging. This helps maintain consistency in the SHG signaling as the scope images deeper into the tissue.
- Once the z-stacks have been generated, process them further in ImageJ/FIJI to create a movie of optical sections in the transverse orientation. To do this, open the ImageJ/FIJI software14 and click on Image > Stacks > Reslice. This will allow us to have a transverse view of the Achilles tendon, revealing the stellate morphology of tenocytes and their orientation in the collagen matrix.
Representative Results
This protocol is useful for characterizing tendon cells and their extracellular matrix (ECM) after injury, during development, or in a mutant condition. With careful dissection and preparation of the sample, z-stack videos can be generated through the tissue in a sagittal orientation (Video 1 and Video 2). By using ImageJ/FIJI to process and reslice the images, a transverse view of the Achilles tendon is created (Videos 3 and Video 4). This reveals the stellate morphology of tenocytes and enables us to observe their unique architecture within the dense collagen matrix.
By utilizing multiphoton microscopy and leveraging the non-centrosymmetric crystalline properties of fibrillar collagen I, high-resolution optical section images that capture both the tendon cells and their matrix were generated. These optical sections are isolated from the z-stacks for both the BHLHE40 +/-; Scx-GFP and BHLHE40 -/-; Scx-GFP mice (Figure 1A,B, respectively). The sections are taken from the original sagittal z-stack created by 2-P imaging and from the re-sliced z-stack generated using ImageJ/FIJI. In addition to this, we can also use this imaging method to analyze any potential differences in collagen organization, cellular morphology, etc., between the two mice.
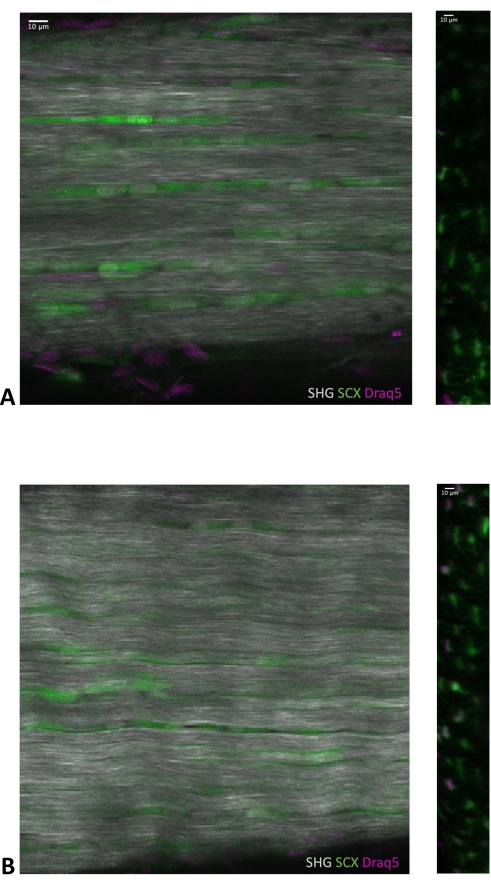
Figure 1: Optical section of BHLHE40 +/-; Scx-GFP and BHLHE40 -/-; Scx-GFP mouse tendon. Z-stack was captured using a multiphoton microscope. (A) The optical sections acquired from the Z-stack show the SHG signal and the GFP+/Draq5+ cells in a BHLHE40 +/-; Scx-GFP (P30, female, 14.68 g) and (B) BHLHE40 -/-; Scx-GFP (P30, female, 12.16 g). Sagittal sections have SHG signal included while transverse sections do not. Samples were counterstained with Draq5. SHG captures the collagen matrix in the tissue. Scale bar = 10 µm. Please click here to view a larger version of this figure.
Video 1: Z-stack videos of the sagittal view of BHLHE40 +/-; Scx-GFP mouse tendon. Z-stack was captured using a two-photon microscope. The video shows the sagittal view of the Achilles tendon. Samples were counterstained with Draq5. SHG captures the collagen matrix in the tissue. Please click here to download this Video.
Video 2: Z-stack videos of the sagittal view of BHLHE40 -/-; Scx-GFP mouse Achilles tendon. Z-stack was captured using a two-photon microscope. The video shows the sagittal view of the Achilles tendon in a BHLHE40 null KO mouse. Samples were counterstained with Draq5. SHG captures the collagen matrix in the tissue. Please click here to download this Video.
Video 3: Z-stack videos of the transverse view of BHLHE40 +/-; Scx-GFP mouse tendon. Z-stack was captured using a two-photon microscope. The video shows the transverse view of the Achilles tendon. Samples were counterstained with Draq5. SHG captures the collagen matrix in the tissue. Please click here to download this Video.
Video 4: Z-stack videos of the transverse view of BHLHE40 -/-; Scx-GFP mouse Achilles tendon. Z-stack was captured using a two-photon microscope. The video shows the transverse view of the Achilles tendon in a BHLHE40 null KO mouse. Samples were counterstained with Draq5. SHG captures the collagen matrix in the tissue. Please click here to download this Video.
Discussion
This article presents a method to prepare, dissect, and image the mouse Achilles tendon, utilizing the non-centrosymmetric crystalline properties of the tendon ECM. Key steps in tissue preparation involve permeabilization for counterstains and ensuring proper tissue placement in a petri dish during imaging. Instead of Draq5, Hoechst 33258 can be used at a 1:100,000 dilution13, but Draq5 is preferred for its high permeability, photostability, and minimal photobleaching. Proper tissue placement is crucial to avoid bubbles between the coverslip and the tendon, as incorrect orientation can affect the resulting images.
Compared to other microscopy methods, second harmonic generation (SHG) microscopy enables the capture of collagen fibrils and tendon cells within the matrix10. Combined with counterstains, this data allows for the analysis of how development, injuries, or mutations affect tendon structure. Generating 3D images of tendon ECM allows the observation of optical sections as they pass through the tendon. While multiphoton technology provides superior tissue penetration, it still has limitations in imaging through the entire Achilles tendon, necessitating additional techniques like sectioning. However, this method enables the production of approximately 150 0.4-micron optical sections with clarity, avoiding tissue perturbation through sectioning.
In summary, this method offers valuable insights into characterizing tendon development, injuries, healing, and aging, by imaging tendon cells and ECM using multiphoton microscopy. Utilizing multiphoton imaging for optical sections and z-stack videos throughout the tissue provides a better understanding of changes in tendon morphology under experimental conditions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Jenna Galloway and the members of Galloway Lab for their support and encouragement in the development and troubleshooting of these protocols.
Materials
| 0.5 M EDTA pH 8.0 | Invitrogen | AM9262 | |
| 2 mL microcentrifuge tubes | USA Scientific | 1620-2700 | |
| 20 mL scintillation vial | Sigma-Aldrich | Z190527-1PAK | |
| 4% Paraformaldehyde | Electron Microscopy Sciences | 50-980-487 | Use PFA ampuole to create 4% PFA solution |
| 6 mm Biopsy Punch Tool | Ted Pella Inc. | 15111-60 | |
| 60 x 15 mm petri dish | |||
| BHLHE40 null knockout or heterozygous mice in a Scx-GFP background | The Jackson Laboratory | JAX ID #029732 | MGI ID #3717419 |
| Coverslips | Fisher | 12-544-F | Can use any coverslip that spans the area of the M20 washer |
| dPBS | Gibco | 14190144 | |
| Draq5 | ABCAM | ab108410 | |
| Fine scissors 21 mm cutting edge | Fine Science Tools | 14060-10 | |
| FVMPE-RS multiphoton laser scanning microscope | Olympus | ||
| Gelfoam Sterile Sponge Size 50 | Pfizer | 00009-0323-01 | |
| INSIGHT X3-OL IR pulsed laser | Olympus | ||
| MaiTai HPDS-O IR pulsed laser | Olympus | ||
| Phosphate-Buffered Saline (1x) | Invitrogen | AM9625 | Dilute 10x PBS in milli-Q water to get 1x solution |
| Stainless steel M20 flat washer | McMaster-Carr | ||
| Triton X-100 | MP Biomedicals | 807426 | Dilute Triton X-100 in dPBS to get 1% solution |
| Vannas spring scissors 4 mm cutting edge | Fine Science Tools | 15018-10 | |
| XLPlan N 25X WMP Lens |
References
- Sharma, P., Maffulli, N. Tendon injury and tendinopathy: healing and repair. The Journal of Bone and Joint Surgery. 87 (1), 187-202 (2005).
- Kadler, K. E., Holmes, D. F., Trotter, J. A., Chapman, J. A. Collagen fibril formation. The Biochemical Journal. 316 (Pt 1), 1-11 (1996).
- Butler, D. L., Grood, E. S., Noyes, F. R., Zernicke, R. F. Biomechanics of ligaments and tendons. Exercise And Sport Sciences Reviews. 6, 125-181 (1978).
- Tsai, S. L., Nödl, M. T., Galloway, J. L. Bringing tendon biology to heel: Leveraging mechanisms of tendon development, healing, and regeneration to advance therapeutic strategies. Developmental Dynamics: An Official Publication of the American Association of Anatomists. 250 (3), 393-413 (2020).
- Subramanian, A., Schilling, T. F. Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development (Cambridge, England). 142 (24), 4191-4204 (2015).
- Lin, T. W., Cardenas, L., Soslowsky, L. J. Biomechanics of tendon injury and repair. Journal of Biomechanics. 37 (6), 865-877 (2004).
- Riley, G. P., Harrall, R. L., Constant, C. R., Chard, M. D., Cawston, T. E., Hazleman, B. L. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Annals of the Rheumatic Diseases. 53 (6), 359-366 (1994).
- Bobzin, L., Roberts, R. R., Chen, H. J., Crump, J. G., Merrill, A. E. Development and maintenance of tendons and ligaments. Development (Cambridge, England). 148 (8), dev186916 (2021).
- Hodgson, R. J., O’Connor, P. J., Grainger, A. J. Tendon and ligament imaging. The British Journal of Radiology. 85 (1016), 1157-1172 (2012).
- Williams, R. M., Zipfel, W. R., Webb, W. W. Interpreting second-harmonic generation images of collagen I fibrils. Biophysical Journal. 88 (2), 1377-1386 (2005).
- Centonze, V. E., White, J. G. Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophysical Journal. 75 (4), 2015-2024 (1998).
- Franchi, M., Trirè, A., Quaranta, M., Orsini, E., Ottani, V. Collagen structure of tendon relates to function. The Scientific World Journal. 7, 404-420 (2007).
- Grinstein, M., Dingwall, H. L., O’Connor, L. D., Zou, K., Capellini, T. D., Galloway, J. L. A distinct transition from cell growth to physiological homeostasis in the tendon. eLife. 8, e48689 (2019).
- Rueden, C. T., Schindelin, J., Hiner, M. C., et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 18, 529 (2017).

.
