Isolation of Viral Replication Compartment-enriched Sub-nuclear Fractions from Adenovirus-infected Normal Human Cells
Summary
We provide a novel strategy to isolate viral replication compartments (RC) from adenovirus (Ad)-infected human cells. This approach represents a cell-free system that can help to elucidate the molecular mechanisms regulating viral genome replication and expression as well as regulation of viral-host interactions established at the RC.
Abstract
During infection of human cells by adenovirus (Ad), the host cell nucleus is dramatically reorganized, leading to formation of nuclear microenvironments through the recruitment of viral and cellular proteins to sites occupied by the viral genome. These sites, called replication compartments (RC), can be considered viral-induced nuclear domains where the viral genome is localized and viral and cellular proteins that participate in replication, transcription and post-transcriptional processing are recruited. Moreover, cellular proteins involved in the antiviral response, such as tumor suppressor proteins, DNA damage response (DDR) components and innate immune response factors are also co-opted to RC. Although RC seem to play a crucial role to promote an efficient and productive replication cycle, a detailed analysis of their composition and associated activities has not been made. To facilitate the study of adenoviral RC and potentially those from other DNA viruses that replicate in the cell nucleus, we adapted a simple procedure based on velocity gradients to isolate Ad RC and established a cell-free system amenable to conduct morphological, functional and compositional studies of these virus-induced subnuclear structures, as well as to study their impact on host-cell interactions.
Introduction
Adenoviruses contain a double-stranded DNA genome that replicates in the infected cell nucleus. When the viral DNA enters the nucleus, it localizes adjacent to PML nuclear bodies1. Following viral early gene expression, the nuclear architecture is dramatically reorganized, inducing formation of viral microenvironments, termed viral Replication Compartments (RC)2. Since adenovirus (Ad) RC are sites where viral genome replication and expression of viral late genes take place, they provide an environment for recruitment of all the necessary viral and cellular factors that participate in these processes. Interestingly, a variety of cellular proteins responsible for the cellular antiviral response, such as the DNA damage response, the innate immune response and tumor suppression are co-opted to these viral sites2. Hence, Ad RC can be considered regulatory hubs that promote efficient viral replication while concomitantly regulating the cellular antiviral response, indicating that these structures are key to the understanding of virus-host cell interactions. Nevertheless, the molecular mechanisms of RC formation, their composition and associated activities are poorly understood.
Adenoviral RC, as well as RC from other DNA viruses that replicate in the nucleus are not associated to membranes, in contrast to cytoplasmic RC3. Moreover, these virus-induced structures are likely to be composed entirely of proteins and nucleic acids. RC formed in cells infected with RNA viruses (usually termed viral factories) have been isolated, taking advantage of their cytoplasmic localization and membrane-bound status, which has facilitated their detailed morphological, functional and biochemical characterization4.
To our knowledge, nuclear viral RC have not been isolated, perhaps due to the complexity of the nuclear architecture and absence of intranuclear membranes that would facilitate their isolation. Their study has relied instead on immunofluorescence microscopy, FISH and transmission electron microscopy. However, despite complications inherent to isolating subnuclear structures, other nuclear domains such as nucleoli and Cajal Bodies have been isolated before 5,6. Since nucleoli and RC are both composed of proteins and nucleic acids, and have a diameter between 0.5 – 5 µm, we hypothesized that RC should also be amenable to isolation. Therefore, in order to more precisely characterize the molecular composition and functions associated to RC, we established a novel method to isolate subnuclear fractions enriched with RC. To this end, we prepared sub-nuclear fractions using velocity gradients and sucrose cushions similar to procedures used to isolate nucleoli7 or other nuclear domains6 and established a cell-free system that allows the study of the molecular composition and associated activities of RC. This technique should therefore advance the understanding of virus-host cell interactions and represents a powerful tool that should also facilitate the detailed analysis of RC from other viruses that replicate in the nucleus and induce formation of replication compartments of similar dimensions to those formed in adenovirus-infected-cells, such as, herpesviruses, papillomaviruses or polyomaviruses.
Protocol
1. HFF Cell Culture and Ad-infection
- Propagate Ad5 WT virus in monolayers of HEK-293 cells and titer as fluorescent forming units (FFU) on HFF cells as described previously8.
- Grow Human Foreskin fibroblasts (HFF) in 10 ml of DMEM/10% Fetal Bovine Serum (FBS) in sterile culture 100 mm dishes at 37 ºC and 5% CO2 in a humidified incubator. Determine the cell number using a Neubauer chamber by counting cells in the four 16-square sets. The cell number per ml is obtained by calculating the average number of cells in the four 16-square sets and multiplying this number by 104. For each time post-infection included in step 1.3, use 1 x 107 HFF cells.
- Mock-infect or infect HFF cells with adenovirus type 5 (Ad5) wild-type (WT) (H5pg41008) in 100 mm tissue culture dishes using 1 ml of Ad5 in DMEM per dish at an MOI of 30 Focus Forming Unit, or FFU, per cell. Incubate for 2 hr in a humidified cell culture incubator at 37 ºC and 5% CO2, carefully rocking the dishes every 15 min to ensure homogeneous distribution of the virus inoculum over the cells. After this time, remove the medium and add fresh DMEM supplemented with 10% FBS and incubate for 16, 24 or 36 hr in a humidified cell culture incubator at 37 ºC and 5% CO2. Proceed to step 2.1.
2. Preparation of Sub-nuclear Fractions Enriched with Adenovirus RC
- Harvest Ad5-infected or mock-infected HFF cells with a cell scrapper and collect the cells in sterile centrifuge tubes. Determine the cell number as in step 1.2. Use 1 x 107 cells for each time post-infection specified in step 1.3.
- Centrifuge the cells at 220 x g, 4 ºC for 5 min.
- Resuspend the cell pellet in ice-cold PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 1.8 mM KH2PO4). Wash cell pellets 3 times using 5 ml of ice-cold PBS per wash. For this purpose, centrifuge the cells at 220 x g, 4 ºC for 5 min, decant and discard the supernatant (SN), and resuspend the cell pellet by gentle pipetting.
- To disrupt the plasma membrane, resuspend the cell pellet in 700 µl of ice-cold hypotonic buffer (10 mM HEPES pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT, 20 µg/ml phenylmethylsulfonyl fluoride (PMSF), a mixture of cysteine, serine, threonine and aspartyl protease inhibitors including 10 µg/ml bovine pancreatic trypsin inhibitor, 10 µg/ml pepstatin A and 10 µg/ml N-acetyl-L-leucyl-L-leucyl-L-argininal) and let the cells swell on ice for 3 hr.
- Lyse the cells using a homogenizer with a loose-fitting A type teflon pestle. Perform 80 dounce strokes and monitor samples every 20 strokes by bright field microscopy to ensure that all cells have been lysed, but that nuclei have not been damaged or ruptured.
- Centrifuge the cell homogenate at 300 x g, 4 ºC for 5 min. Store the SN as the cytoplasmic fraction at -20 ºC in a centrifuge tube.
- To remove cellular debris from nuclei, resuspend the pellet in 750 µl of solution 1 (S1) (0.25 M sucrose, 10 mM MgCl2 20 µg/ml PMSF, a mixture of cysteine, serine, threonine and aspartyl protease inhibitors including 10 µg/ml bovine pancreatic trypsin inhibitor, 10 µg/ml pepstatin A and 10 µg/ml N-acetyl-L-leucyl-L-leucyl-L-argininal) and layer over an equal volume of solution 2 (S2) (0.35 M sucrose, 0.5 mM MgCl2, 20 µg/ml PMSF, a mixture of cysteine, serine, threonine and aspartyl protease inhibitors including 10 µg/ml bovine pancreatic trypsin inhibitor, 10 µg/ml pepstatin A and 10 µg/ml N-acetyl-L-leucyl-L-leucyl-L-argininal). Centrifuge at 1,400 x g, 4 ºC for 5 min.
- Discard the SN using a micropipette. Avoid disturbing the pellet.
- Resuspend the pellet containing isolated nuclei in 750 µl of S2.
- To isolate subnuclear fractions enriched with adenovirus RC (RCf), sonicate resuspended nuclei with an ultrasonic bath, using two 5 min pulses, or until all nuclei are lysed as observed by bright field microscopy. Use ice in ultrasonic bath as needed to keep samples at or below 4 °C.
- Layer the sonicated nuclei over an equal volume of solution 3 (S3) (0.88 M sucrose, 0.5 mM MgCl2) and centrifuge at 3,000 x g, 4 ºC for 10 min. Store the 1.5 ml supernatant as the nucleoplasmic fraction (Npl) at -70 ºC. Resuspend the pellet in 700 µl of S2 and store as RCf at -70 ºC.
3. Western Blot Analyses of RCf
NOTE: For Western Blot analysis of Npl and RCf fractions set aside 640 µl for Npl and 300 µl for RCf from the total volume obtained in step 2.11.
- Mix 15 µl of each subnuclear fraction in Laemmli buffer 2x (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromophenol blue, 0.125 M Tris HCl pH 6.8) and boil at 95 ºC for 5 min. Load the samples in a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) denaturing gel and separate proteins for 1.5 hr, at 20 milliamperes (mA).
- Transfer proteins by electroblotting for 1.5 hr at 400 mA onto polyvinylidene difluoride (PVDF) membranes.
- Block membrane for 2 hr at RT using 3% nonfat dry milk in PBS.
- Incubate O/N at 4 ºC with primary antibody against viral E2A DNA-binding protein (B6-89) at a 1:500 dilution in PBS/0.3% nonfat dry milk.
- Wash membrane 3 times with PBS/0.1% Tween 20 for 10 min.
- Incubate the membrane with mouse anti IgG secondary antibody coupled to horse-radish peroxidase (HRP) at a 1:10,000 dilution in PBS for 2 hr at RT.
- Wash the membrane 3 times with PBS/0.1% Tween 20 for 10 min.
- Develop the membrane using enhanced chemiluminescence and X-ray films.
4. Viral DNA Detection in RCf
NOTE: For DNA isolation from both Npl and RCf fractions, use 210 µl for Npl and 100 µl for RCf of the total volume obtained in step 2.11.
- Incubate sub-nuclear fractions for 1 hr at 55 ºC with 1 mg/ml of proteinase K and 0.5% Tween 20.
- Inactivate proteinase K by incubating the samples for 10 min at 95 °C.
- Centrifuge the samples at 20,000 x g, at RT for 2 min.
- Collect the SN. Precipitate the DNA with 1/10 volume of 3M sodium acetate and one volume of isopropanol, O/N at 4 °C.
- Centrifuge the samples at 20,000 x g, at RT for 10 min.
- Discard the SN using a micropipette. Wash the pellet with 70% ethanol and centrifuge at 20,000 x g, 4 °C for 5 min.
- Resuspend the DNA in 10 µl of 10 mM Tris-HCl pH 7.4
- Quantify DNA using a spectrophotometer by measuring the optical density (OD) at 260 nm.
- To amplify viral DNA, use 100 ng of DNA from each subnuclear fraction in a standard PCR reaction using Taq DNA polymerase in 25 µl of reaction volume with primers that allow the amplification of a region within the Major Late transcription unit, from nucleotide 7273 to nucleotide 7353 (81 bp) (Fw: GAGCGAGGTGTGGGTGAGC; Rv: GGATGCGACGACACTGACTTCA). Use the following cycle conditions: Initial denaturation for 3 min at 95 ºC, followed by 20 cycles of amplification (1 min at 95 ºC, annealing for 1 min at 62 ºC and extension for 30 sec at 72 ºC) and a final extension step for 3 min at 72 ºC.
- Run the PCR product in 2% agarose gels, at 90 V. Stain with ethidium bromide at 0.5 µg/ml final concentration and visualize DNA to corroborate viral DNA enrichment in the RCf. Handle ethidium bromide with extreme caution and always make sure to wear gloves, as this compound is a potent mutagen. Dispose ethidium bromide according to institutional guidelines.
5. Late Viral mRNA Detection in RCf
NOTE: For RNA isolation from both Npl and RCf fractions, use 640 µl for Npl and 300 µl for RCf from the total volume obtained in step 2.11.
- Isolate RNA from subnuclear fractions using Trizol according with the manufacturer’s instructions. Resuspend the RNA in 50 µl of DEPC (diethilpyrocarbonate)-treated water.
- Quantify the RNA using a spectrophotometer to measure the OD at 260 nm (approximately 3 µg are obtained). Store the RNA in 50 ng/µl aliquots at -70 ºC.
- Check purified RNA for the absence of DNA contamination by performing a control PCR reaction in the absence of reverse transcriptase (RT), using 5 ng of total RNA and the cycle conditions described in step 4.9.
- If DNA contamination is absent no amplicon should be produced. If DNA contamination is present, incubate the samples with 10 U DNase I, 10 U of Rnase inhibitor, 0.1 M Tris-HCl pH 8.3, 0.5 M KCl and 15 mM MgCl2 for 30 min at 37 ºC. Proceed to re-isolate RNA as in step 5.1.
- To analyze viral mRNA associated to RCf, amplify 100 ng of RNA from each subnuclear fraction by RT-PCR using primers designed to detect adenoviral late mRNA from different gene families (Table 1).
- For reverse transcription (RT), use 100 ng of RNA, from each subnuclear fraction in a standard RT reaction using M-MuLV RT enzyme in 20 µl of reaction volume for 1 hr at 42 ºC and 10 min at 70 ºC.
- For amplification, use 1 µl of the cDNA in PCR reactions, as in step 4.9. Use the following cycle conditions: Initial denaturation for 3 min at 95 ºC, followed by 25 cycles of amplification (1 min at 95 ºC, annealing for 1 min at the temperature specified in Table 1 and extension for 30 sec at 72 ºC) and a final extension step for 3 min at 72 ºC.
- Run the RT-PCR products in 2% agarose gels, at 90 V. Stain gels with ethidium bromide at 0.5 µg/ml final concentration and visualize to corroborate viral late mRNA association to the RCf. Handle ethidium bromide as indicated in step 4.10.
6. Immunofluorescence Visualization of RCf
NOTE: Carry-out this procedure under a laminar flow cabinet to avoid contamination of the samples with any dust particles, and filter all solutions before use.
- Spot 5 µl of the RCf fraction obtained in step 2.10 directly on a silane-coated slide.
- Let the spot dry for about 5 min at RT.
- Re-hydrate by slowly and indirectly pipetting 500 µl of PBS in a drop beside the RCf spot and letting it flow by tilting the slide. Drain off the excess PBS from the side.
- Block by covering the sample with 500 µl of PBS/5% bovine serum albumin (BSA) for 2 hr at RT.
- Wash the slide 3 times by gently and indirectly pipetting 500 µl of PBS onto the sample. Tilt the slide to drain off the excess PBS.
- Incubate the sample with primary antibody against viral E2A DNA-binding protein (B6-89) at a 1:500 dilution in PBS for 2 hr at RT. Cover the spotted sample with 20 µl of the antibody solution and incubate in a humid chamber to avoid drying.
- Wash the sample 3 times with PBS/0.02% Tween 20 by gently and indirectly pipetting 500 µl of PBS onto the sample. Tilt the slide to drain off the excess wash solution.
- Incubate the samples with mouse anti IgG secondary antibody coupled to a fluorophore that is excited at 488 nm at a 1:2,000 dilution in PBS, for 1 hr at 4 ºC. Cover the spotted sample with 20 µl of the antibody solution and incubate in a humid chamber to avoid drying.
- Wash the sample 3 times with PBS/0.02% Tween 20 by gently and indirectly pipetting 500 µl of PBS onto the sample. Tilt the slide to drain off the excess wash solution.
- Cover the spotted sample on the slide with a coverslip using 2 µl PBS/10% glycerol as mounting medium. Seal with clear nail polish and store al -20 ºC.
- Analyze the samples using a fluorescence microscope, with a 63x objective and a 1.4 Numeric Aperture (NA) at a wavelength of 488 nm.
Representative Results
Since viral replication compartments (RC) are subnuclear viral-induced structures composed of proteins and nucleic acids, similar to other nuclear domains, they proved to be amenable to isolation by velocity gradients based on biochemical features. Critical steps in the fractionation protocol are illustrated in Figure 1. At each step the samples need to be monitored by bright field microscopy to ensure integrity of the different sub-cellular fractions. For example, when swelling the cells, incubation time in the hypotonic buffer needs to be standardized in order to swell the cytoplasm avoiding damage to the nuclei. After cell homogenization, intact nuclei, free of cytoplasmic components including endoplasmic reticulum membranes, need to be obtained. Also, sonication time needs to be standardized in order to rupture the nuclear membrane of all cells without disrupting RC.
After obtaining the sub-nuclear fractions, key controls need to be included to determine the association and enrichment of bona fide RC markers in the RCf. Adenoviral RC are commonly visualized in infected cells by immunofluorescence using antibodies against the viral E2A-72K protein (DBP). DBP is a viral protein that participates directly in viral genome replication; therefore, the presence of DBP in particles enriched in the RCf demonstrates the direct association of this viral protein with the isolated particles, as shown in Figure 1. Furthermore, detection of DBP in RCf by Western Blot confirms the association of this protein to the isolated RC. In Figure 2 it is shown that by late times post-infection (36 hpi), DBP is enriched in the RCf compared to the nucleoplasmic fraction (Npl), demonstrating that RC obtained with this procedure at different times post-infection reflect the expected temporal pattern of DBP association to RC. An essential viral component of RC is the viral DNA itself. In experiments like that shown in Figure 3 we demonstrate that increasing amounts of viral DNA associate with RCf as the replication cycle of the virus progresses, indicating that, as for DBP, the temporal pattern of DNA replication in these fractions can also be studied.
Besides containing viral proteins and the viral genome, RC are also sites of viral late gene expression. In Figure 4 we present representative results of experiments designed to measure by RT-PCR the level of various species of viral late mRNA and their segregation between RCf and Npl at 36 hpi. Viral late mRNA are synthesized in RC and their postranscriptional processing initiates in these sites; later these viral mRNA dissociate from RC, are liberated to the nucleoplasm, and are subsequently exported to the cytoplasm. The total pool of mature viral late mRNA (ML mRNA) was measured using primers that amplify an exon junction of the tripartite leader, a sequence that constitutes the 5’ of all adenoviral late mRNA (Figure 4A). Exon junctions in the mRNA of specific mature transcripts of the L2, L4 and L5 families were also measured. It has been established that as the late phase of the viral replication cycle progresses, increasing amounts of viral late mRNA are exported to the cytoplasm; however, by late times production of the L5 mRNA species increases further in comparison with the other late mRNA families. In the representative results sown in Figure 4 an approximately 2.5-fold difference in ML mRNA is observed in Npl compared with RCf, as expected. Interestingly when we compare mRNA from specific families, both L2 and L4 mRNA appear to be distributed in similar levels between both subnuclear fractions (Figure 4B and C, respectively), while in contrast, the L5 mRNA showed an almost 2-fold increase in Npl compared to RCf. These results suggest a differential pattern in the synthesis and liberation of the different viral late mRNA species from adenoviral RC (as has been suggested before10). Significantly, these results also demonstrate that precise measurements of the different steps in the biogenesis of the viral mRNA can be performed using isolated RC with this novel procedure.
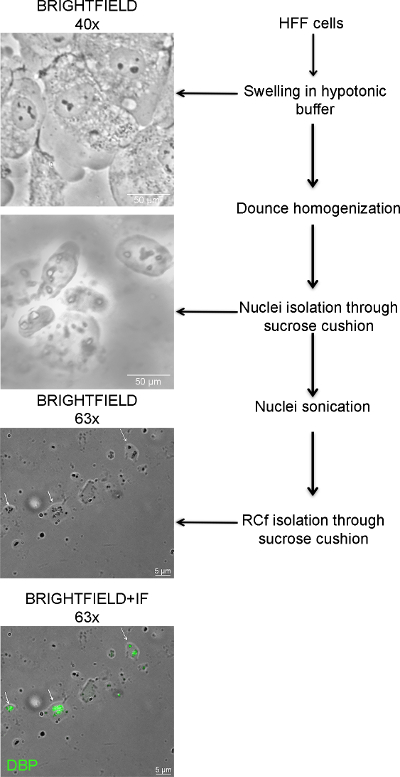
Figure 1. Bright Field Microscopy of Different Steps during the Fractionation Procedure. During the isolation of RC, the samples need to be monitored by an optical microscope to ensure integrity of sub-cellular fractions. The figure shows the steps used in the procedure to obtain RCf, from the swelling of HFF cells, to separation of nuclei and isolation of RCf through sucrose cushions. 40x micrographs: scale bar 50 µm; 63x micrographs: scale bar 5 µm; DBP is shown in green. Please click here to view a larger version of this figure.
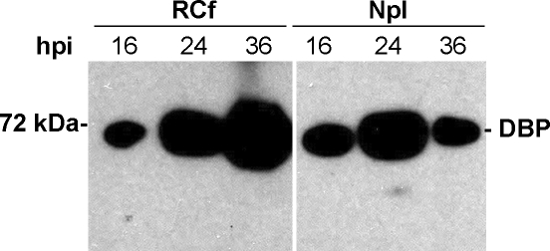
Figure 2. Western Blot against DBP. The samples are analyzed by SDS-PAGE and processed for western blot against DBP, a bona fide marker of viral RC. To determine the enrichment of the protein in RCf, it is useful to compare the presence and relative abundance of DBP in both RCf and Npl at different times post-infection. In HFF cells 16 hpi represents an early time during the adenoviral replication cycle; 24 hpi marks the transition to the late phase of infection as viral DNA synthesis begins; 36 hpi represents a late time post-infection. The expected molecular mass of DBP is shown. Please click here to view a larger version of this figure.
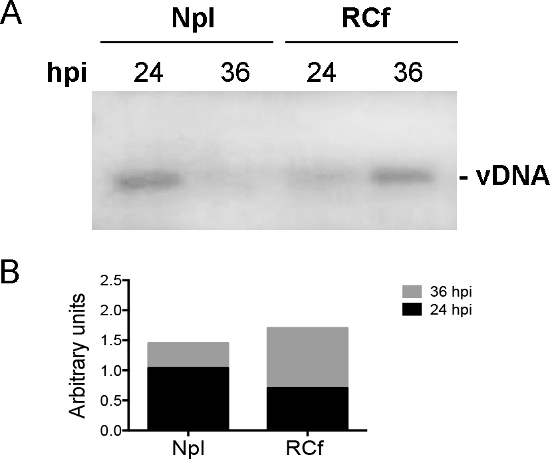
Figure 3. PCR Assay to Detect Viral DNA (vDNA) in RCf. DNA was purified from RCf and Npl at 24 and 36 hpi. Viral DNA was amplified by PCR using primers specific for the viral genome. The graph shows the enrichment of viral DNA in RCf at 36 hpi. Please click here to view a larger version of this figure.
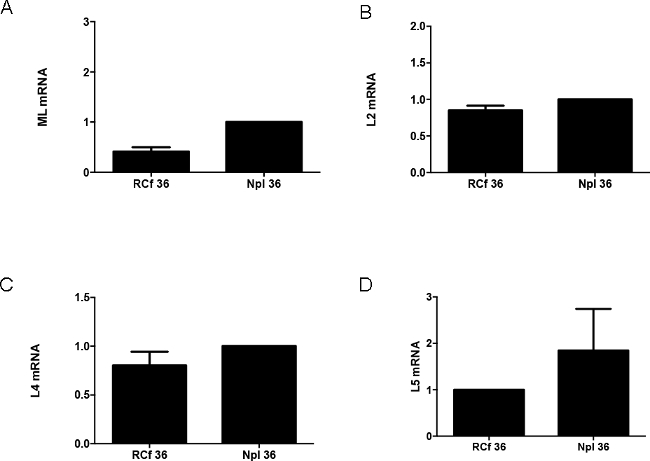
Figure 4. Analysis of Viral Late mRNA. RNA was isolated from RCf and Npl and analyzed by RT-PCR to detect specific viral late mRNA. (A) Total Viral Late mRNA (ML: Major Late); (B) mRNA from the L2 Family; (C) mRNA from the L4 Family; (D) mRNA from the L5 Family. Values represent mean ± standard deviation of triplicate samples. Please click here to view a larger version of this figure.
| Name | Forward primer sequence (5'-3') | Reverse primer sequence (5'-3') | Annealing temperature |
| ML mRNA | GCCTCCGAACGGTACTCCGCC | CGCCACGGTGCTCAGCCTACC | 60 ºC |
| L2 mRNA | GTCACAGTCGCAAGATGTCCAAGC | GCAACGCCAGCATGTCCTTATGC | 58 ºC |
| L4 mRNA | CCTCCGAACGGTACTCCGC | CCTTGCTCATCTTGCGACTGTG | 58 ºC |
| L5 mRNA | GTCACAGTCGCAAGATGAAGCG | GGTAACTAGAGGTTCGGATAGGCG | 60 ºC |
Table 1. Primers Used for Viral Late mRNA Amplification. ML mRNA: these primers allow the amplification of a region within the tripartite leader, the 5’ sequence that is common to all viral late mRNA; L2 mRNA primers allow the amplification of a specific region within the pV mRNA; L4 mRNA primers allow the amplification of a specific region within the 100 K mRNA; L5 mRNA allows the amplification of a region within the fiber mRNA. The sequence and annealing temperatures for each primer are shown.
Discussion
In order to elucidate the molecular mechanisms that govern regulation of cellular activities by viral infection understanding the composition and activities associated with RC would be instrumental. Therefore, to make a detailed analysis of RC, we established a cell-free system that takes advantage of the size and biochemical composition of these virus-induced structures, to isolate subnuclear fractions enriched with RC using a simple procedure that relies on velocity gradients with sucrose cushions. Critical steps of the procedure that require standardization depending on the cell type used are: i) standardization of the times used for cell swelling to avoid disruption of nuclei; ii) formation of the sucrose gradients so that fractions can be properly separated; iii) constant monitoring of the samples throughout the procedure by bright field microscopy; iv) sonication time and intensity to ensure that all nuclei are lysed but RC are not disrupted; v) all fractionation steps should be carried out on ice to avoid disruption of nuclear structures.
Limitations of the technique that can be encountered or can be anticipated are: i) that nucleoli and perhaps other subnuclear structures or domains that share similar dimensions and biochemical composition are coisolated in the RC fraction, making the relative abundance of replication compartments versus other similar nuclear bodies variable depending on the virus, cell type and time post-infection used for their isolation; ii) it is possible that just as the size and composition of RC changes as the replication cycle of the virus progresses, the compactness of the RC particles may also change, making it necessary to perform experiments designed to determine detailed ultrastructural changes of the RC particles at different times post-infection to evaluate not only changes in the particles composition and activities, but whether their stability may vary. While these issues may limit the characterization of RC composition and activities at various different times post-infection, as less stable RC obtained by this procedure may not recapitulate their counterpart in the infected cell nucleus, such limitations may be overcome by using higher multiplicities of infection or by determining the times post-infection at which viral RC have attained a minimum size and abundance in the infected cell nucleus. These potential limitations notwithstanding, the technique reported here provides several advantages over the analysis that has hitherto solely relied on microscopy; namely that the precise biochemical and functional analysis of RC can be performed using this novel approach.
We show here that the RC-fractions (RCf) obtained are enriched with bona fide RC markers, such as the viral E2-72K DBP protein, viral DNA and RNA. Using biochemical analysis by immunoblotting and PCR, we have confirmed that this approach facilitates the detailed analysis of components and activities associated to RC and should help unravel molecular mechanisms that regulate virus-host cell interactions in the infected-cell nucleus.
It is well established that DNA replication, transcription and post-transcriptional processing of viral late mRNA are closely associated to viral replication compartments, events that lead to viral progeny production2,11,12. In adenovirus-infected cells the onset of viral DNA replication marks the transition to the late phase of infection and results in the activation of the Major Late promoter, which directs the production of high quantities of viral late transcripts. However, the L4 genes are expressed through a novel promoter (L4P) that is independent from the MLP13,14. Besides this complex program of late promoter activation, the selection of 3’ splice sites for longer introns is progressively favored as the late phase proceeds. In addition, production of these transcripts is regulated by their selective release from the site of synthesis and by selective export to the cytoplasm.
However, regulation of the late promoters and molecular events that lead to the selective production of viral late mRNA is incompletely understood. Therefore, as a proof of concept for the procedure reported here, we demonstrate that viral DNA and viral late mRNA can be isolated in RCf, suggesting that these subnuclear fractions can be used to determine the pattern of synthesis and partitioning of different species of viral late mRNA, hence allowing the use of quantitative techniques and potentially advancing the detailed study of the complex pattern of viral late mRNA biogenesis.
Furthermore, this technique can be used to study the molecular mechanisms that are responsible for the efficient replication of the viral genome, regulation of the complex program of viral gene transcription and cellular factors that are co-opted in RC, as well as morphological and ultrastructural studies of these viral induced structures. Moreover this system should be easily adapted to isolate RC from other nuclear replicating viruses, and should help unravel viral mechanisms that govern virus-cell interactions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from CONACyT-SEP (SEP-2008-84582; CB-2011-01-168497) and Promep-SEP for R.A.G.; P.H. received a scholarship from CONACyT (447442).
Materials
| DMEM | Gibco | 12100-046 | Warm in 37 ºC water bath before use |
| Fetal Bovine Serum | Gibco | 12484-028 | |
| Sucrose, Ultra Pure | Research Organics | 0928S | Prepare a 2.55 M stock solution and store at 4 ºC |
| Dounce homogenizer | Kontess Glass Company | 884900-0000 | |
| Branson 1800 Ultrasonic Bath | Branson | Z769533 SIGMA | Turn on 15 min before use. |
| Peroxidase AffiniPure F(ab')₂ Fragment Goat Anti-Mouse IgG (H+L) | Jackson ImmunoResearch | 115-036-003 | Use at a 1:10,000 dilution in PBS/0.03% non-fat milk |
| Goat anti-Mouse IgG1 Secondary Antibody, Alexa Fluor 488 conjugate | Life Technologies | A-21121 | Use at a 1:2,000 dilution in PBS |
| Silane-Prep Slides | Sigma | S4651-72EA | Open in a laminar flow cabinet |
| SuperSignal West Pico Chemiluminescent Substrate | Pierce ThermoScientific | 34080 |
References
- Doucas, V., et al. Adenovirus replication is coupled with the dynamic properties of the PML nuclear structure. Genes & Dev. 10, 196-207 (1996).
- Schmid, M., Speiseder, T., Dobner, T., Gonzalez, R. A. DNA virus replication compartments. J. Virol. 88, 1404-1420 (2014).
- Boon, J. A., Diaz, A., Ahlquist, P. Cytoplasmic viral replication complexes. Cell host microbe. 8, 77-85 (2010).
- Paul, D., Hoppe, S., Saher, G., Krijnse-Locker, J., Bartenschlager, R. Morphological and biochemical characterization of the membranous hepatitis C virus replication compartment. J. Virol. 87, 10612-10627 (2013).
- Busch, H., et al. Isolation of Nucleoli. Exp Cell Res. 24, 150-163 (1963).
- Lam, Y. W., Lyon, C. E., Lamond, A. I. Large-scale isolation of Cajal bodies from HeLa cells. Mol. Biol. Cell. 13, 2461-2473 (2002).
- Lam, Y. W., Trinkle-Mulcahy, L., Lamond, A. I. The nucleolus. J Cell Sci. 118, 1335-1337 (2005).
- Groitl, P., Dobner, T. Construction of adenovirus type 5 early region 1 and 4 virus mutants. Methods Mol Med. 130, 29-39 (2007).
- Reich, N. C., Sarnow, P., Duprey, E., Levine, A. J. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology. 128, 480-484 (1983).
- Leppard, K. N. Selective effects on adenovirus late gene expression of deleting the E1b 55K protein. J Gen Virol. 74 (Pt 4), 575-582 (1993).
- Gonzalez, R., Huang, W., Finnen, R., Bragg, C., Flint, S. J. Adenovirus E1B 55-kilodalton protein is required for both regulation of mRNA export and efficient entry into the late phase of infection in normal human fibroblasts. J. Virol. 80, 964-974 (2006).
- Castillo-Villanueva, E., et al. The Mre11 Cellular Protein Is Modified by Conjugation of Both SUMO-1 and SUMO-2/3 during Adenovirus Infection. ISRN Virology. 2014, 14 (2014).
- Morris, S. J., Scott, G. E., Leppard, K. N. Adenovirus late-phase infection is controlled by a novel L4 promoter. J. Virol. 84, 7096-7104 (2010).
- Wright, J., Leppard, K. N. The human adenovirus 5 L4 promoter is activated by cellular stress response protein p53. J. Virol. 87, 11617-11625 (2013).

