Isolation of Physiologically Active Thylakoids and Their Use in Energy-Dependent Protein Transport Assays
Summary
We present protocols herein for high-yield isolation of physiologically active thylakoids and protein transport assays for the chloroplast twin arginine translocation (cpTat), secretory (cpSec1), and signal recognition particle (cpSRP) pathways.
Abstract
Chloroplasts are the organelles in green plants responsible for carrying out numerous essential metabolic pathways, most notably photosynthesis. Within the chloroplasts, the thylakoid membrane system houses all the photosynthetic pigments, reaction center complexes, and most of the electron carriers, and is responsible for light-dependent ATP synthesis. Over 90% of chloroplast proteins are encoded in the nucleus, translated in the cytosol, and subsequently imported into the chloroplast. Further protein transport into or across the thylakoid membrane utilizes one of four translocation pathways. Here, we describe a high-yield method for isolation of transport-competent thylakoids from peas (Pisum sativum), along with transport assays through the three energy-dependent cpTat, cpSec1, and cpSRP-mediated pathways. These methods enable experiments relating to thylakoid protein localization, transport energetics, and the mechanisms of protein translocation across biological membranes.
Introduction
Nearly all of the proteinaceous machinery responsible for proper chloroplast function must be translocated from the cytosol1. At the chloroplast envelopes, protein substrates are imported through the translocon of the outer membrane (TOC) and the translocon of the inner membrane (TIC)2. Further targeting to the thylakoid membrane and lumen occurs through the twin arginine translocation (cpTat)3, the secretory (cpSec1)4, the signal recognition particle (cpSRP)5, and the spontaneous insertion pathways6. A method for the high-yield isolation of physiologically active chloroplasts and thylakoid membranes is necessary to measure the energetics and kinetics of a translocation event, to understand the diverse transport mechanisms in each pathway, and to localize a particular protein substrate of interest to any of the six distinct compartments of the chloroplast.
The isolation of membranes from the chloroplast offers better experimental control over environmental factors (such as salt and substrate concentrations, the presence of ATP/GTP, and pH conditions) that affect the measurement of transport energetics and kinetics. This in vitro environment lends itself to the exploration of mechanistic details of translocation for the same reasons. In addition, while predictive software for localization of chloroplast proteins has improved7,8, in vitro transport assays provide a quicker method for confirmation over microscopy-based fluorescent assays that require a genetically encoded fluorescent tag, plant transformation and/or specific antibodies. Here, we present protocols for chloroplast and thylakoid isolations from peas (Pisum sativum), as well as for transport assays optimized for each of the energy-dependent thylakoid translocation pathways.
Protocol
1. Initial Materials
- Soak approximately 55 g of peas for 3 hours in 400 mL of distilled water, and then sow in a plastic tray (35 cm x 20 cm x 6 cm) in soil covered with thin layer of vermiculite.
- Grow the tray of peas at 20 °C under 12/12 h light/dark (50 µE/m2s) cycle for 9 to 15 days.
- Prepare protein substrate according to a preferred method.
Note: We have prepared protein substrates using a variety of methods, including 1) in vitro transcription from purified plasmids followed by translation using wheat germ extracts or rabbit reticulocyte lysates in the presence of [3H]-leucine or [35S]-methionine, 2) in vitro translation using a coupled transcription/translation kit such as the TnT kits from Promega, with radiolabeling as above, and 3) purification of overexpressed protein in E. coli. Radioactive in vitro translation products are generally quenched with 50 mM cold amino acid prior to use in transport reactions. Each of these methods typically requires some optimization for each new protein substrate. For an example of a coupled in vitro system, see Ling and Jarvis (2016)9.
2. Chloroplast Isolation and Quantification
Note: The first step in preparation of thylakoids is the isolation of intact chloroplasts10. All materials should be kept cold during preparation. Resuspension of chloroplasts should be handled gently, as breakage at this step can severely limit subsequent thylakoid yield.
- Prepare following solutions beforehand.
- 2x Grinding Buffer (2xGB): 100 mM K-HEPES pH 7.3, 660 mM sorbitol, 2 mM MgCl2, 2 mM MnCl2, 4 mM EDTA, 0.2% BSA.
- 2x Import Buffer (2xIB): 100 mM K-Tricine or K-HEPES pH 8.0, 660 mM sorbitol, 8 mM MgCl2.
Note: Concentrations of MgCl2 ranging from 3 mM to 10 mM have been used without observable differences.
- Prepare a continuous density gradient by centrifuging a mixture of 15 mL of Percoll and 15 mL of 2xGB at 30,900 g in a fixed angle rotor for 30 min at 4 °C with the brake off.
- Harvest approximately 25 g of 9-15 day old peas and homogenize in 95 mL of 1xGB. Either a blender with sharpened blades or a Polytron have been used successfully. Filter this mixture through at least 2 layers of Miracloth in a funnel.
Note: Limiting the amount of stem tissue is not necessary but can make the homogenization process easier, thereby reducing chloroplast breakage that can occur with prolonged blending. - Pellet at 3,000 g in a swinging bucket rotor for 5 min at 4 °C, and gently resuspend in 1 mL of 1xGB. A paintbrush may be the most gentle resuspension technique.
- Carefully layer the green suspension on top of the Percoll gradient and centrifuge at 8,000 g in a swinging bucket rotor for 10 min at 4 °C with the brake off.
- Carefully harvest the lower green band containing intact chloroplasts into a fresh tube. Spin the recovered intact chloroplasts at 1,800 g in a swinging bucket rotor for 5 min at 4 °C, discard the supernatant and gently resuspend the pellet in 1xIB. Repeat this wash step once more, resuspending in 30 mL of 1xIB each time, with the final resuspension in 1 mL of 1xIB.
- To quantify chlorophyll (Chl) content, mix 10 µL of fully resuspended chloroplasts with 5 mL of 80% acetone and filter through Whatman 1 filter paper (nominal thickness 180 µm). Record the absorbance at 720 nm, 663 nm, and 645 nm in a spectrophotometer and calculate the Chl concentration using the following formula11:
[Chl] mg/mL = [40.2*(A663 – A720) + 101.4*(A645 – A720)]*0.1 - Adjust chloroplasts to 1 mg/mL Chl equivalents with 1xIB.
3. Isolation of Thylakoids
Note: Thylakoids are prepared by hypotonic lysis of intact chloroplasts. This is achieved by exposing the chloroplasts to a hypotonic buffer lacking sorbitol. Isolated thylakoids can be used for assaying any of the translocation pathways, but stromal extract (SE) must also be isolated during this preparation if either cpSec1 or cpSRP pathways are to be investigated.
- Prepare ahead of time the following solutions.
- Hypotonic Lysis Buffer: 50 mM K-Tricine or K-HEPES pH 8.0, 8 mM MgCl2.
- 2x Crude Stroma Buffer (for concentration of SE): 100 mM K-HEPES pH 8.0, 660 mM sorbitol, 8 mM MgCl2.
- Laemmli Sample Buffer (for SDS-PAGE, supplemented with EDTA): 125 mM Tris pH 6.8, 10% SDS, 20% glycerol, 0.05 mg/mL bromophenol blue, 10% β-mercaptoethanol, 10 mM EDTA.
- If SE recovery is not necessary, centrifuge the intact chloroplasts at 1,000 g in a swinging bucket rotor for 6 min at 4 °C.
- Discard the supernatant and resuspend to 1 mg/mL in Hypotonic Lysis Buffer. Incubate on ice in the dark for 10 min, with occasional mixing.
- Recover thylakoids by centrifuging at 3,200 g in swinging bucket rotor for 8 min at 4 °C. Wash the thylakoids twice, resuspending with 1 to 2 mL of lysis buffer each time. Resuspend with 1 mL of 1xIB and requantify Chl as above in the chloroplast isolation protocol.
4. Stromal Extract Recovery and Concentration
- If SE recovery is necessary, divide the intact chloroplasts into 600 µL aliquots in 1.5 mL microcentrifuge tubes and centrifuge each tube at 1,000 g in swinging bucket rotor for 6 min at 4 °C.
Note: This volume is convenient when using 1.5 mL microcentrifuge tubes, which helps to prevent disturbance of the eventual pellet for residual membranes.- Resuspend to 1 mg/mL Chl in Hypotonic Lysis Buffer and incubate on ice in the dark for 10 min, as in step 3.2.1.
- Centrifuge each tube at 3,200 x g in swinging bucket rotor for 8 min at 4 °C and transfer the light green or yellow supernatant following chloroplast lysis to a new microcentrifuge tube with an equal volume of 2x Crude Stroma Buffer.
- Wash thylakoids with 2 volumes of lysis buffer (approximating 0.5 mg/mL) and collect by centrifugation at 3,200 g in swinging bucket rotor for 8 min at 4 °C. Resuspend to 1 mg/mL Chl in 1xIB on ice.
- Centrifuge crude stroma at 42,000 g in a fixed angle rotor for 30 min at 4 °C to remove residual membranes. Immunoblotting for outer and inner envelope proteins can be used to verify the purity of the supernatant.
- Collect 95% of the volume from each tube without disturbing the small yellow-green pellet. Note the volume taken from each tube.
- To prepare the concentrated stromal extract (SE), pool the collected supernatants and concentrate five-fold using a 4 mL 30 kDa MWCO concentrator.
Note: SE prepared in this manner is equivalent to stroma derived from chloroplasts at 2.5 mg/mL Chl. If necessary, SE can be concentrated ten-fold to 5 mg/mL Chl equivalents. SE will be golden in color and viscous. In the lab’s Legend RT centrifuge with swinging bucket rotor, this requires about 10 min per mL concentrated.
5. Transport through the cpTat Pathway
Note: Unlike the cpSec1 or cpSRP, the cpTat pathway does not require soluble components or exogenously added energy sources; only the light-driven proton motive force is necessary3. Therefore, only isolated thylakoids and substrate protein are required for the assay. Typical substrates are intermediate forms of the 17 kDa (as seen in Figure 1) and 23 kDa subunits of the oxygen evolving complex, iOE17 and iOE23, respectively, but precursor forms, prOE17 and prOE23, can also be successfully transported. Precursor forms have the entire bipartite N-terminal targeting sequence, while intermediate forms have only the thylakoid targeting sequence.
- Prepare cpTat transport mix with 0.33 mg/mL Chl thylakoids, 5 mM ATP from a stock prepared in 1xIB, and 8 mM dithiothreitol (DTT) from a stock prepared in 1xIB, on ice. ATP is not strictly required for this reaction if conducted under illumination.
- Initiate transport by introducing 1:30 volume by volume substrate protein in the transport mix. If substrate protein is not prepared in 1xIB, prepare a 1:1 dilution with 2xIB first, then use 1:30 volume by volume of the diluted substrate in the transport mix.
Note: Concentrations of substrate will vary based on the preparation method. Typically, in vitro preparations will allow for nanomolar to micromolar amounts, while overexpression will allow for micromolar to millimolar amounts. Detection methods must also be considered, as autoradiography and fluorography are more sensitive than immunoblotting. - Illuminate the suspension in 80 – 100 µE/m2s of photosynthetically active radiation (PAR) for 10 min at room temperature. If transport kinetics are required, pre-equilibrate both thylakoids and reaction mix to room temperature and illuminate for up to 30 min.
- Stop the transport reaction with an 8-fold dilution of ice-cold 1xIB and recover thylakoids by centrifugation at 3,200 g in microcentrifuge for 5 min at 4 °C.
- Remove residual substrate that has not been transported, resuspend the thylakoid pellet in 120 µL of 1xIB and digest external protein by addition of 6 µL of 2 mg/mL thermolysin and 10 mM CaCl2 in 1xIB.
- Incubate the protease reaction on ice for 40 min, and then quench the protease by doubling the volume with 25 mM EDTA prepared in 1xIB.
- Recover thylakoids by centrifuging at 3,200 g in a microcentrifuge for 5 min at 4 °C. Wash recovered thylakoids in 120 μL of 5 mM EDTA in 1xIB and transfer to a new tube.
- Centrifuge at 3,200 g in a microcentrifuge for 5 min at 4 °C. Resuspend in an appropriate volume of Laemmli Sample Buffer supplemented with 10 mM EDTA. A volume of 40 µL is usually enough to detect substrate on an SDS-PAGE gel and conserves sample for repeat gels when necessary.
- Place samples in a boiling water bath for 10 min and analyze by SDS-PAGE. Autoradiography, fluorography, or Western blotting of non-native or epitope-tagged proteins have been successfully used for detection of transported proteins.
6. Transport through the cpSec1 Pathway
Note: Transport through the cpSec1 translocon requires the stromal protein cpSecA112,13, which can be procured via overexpression in E. coli14,15 or recovered by concentrating stroma during thylakoid isolation. A typical substrate is the 33 kDa subunit of the oxygen evolving complex (prOE33), as seen in Figure 2.
- Prepare the cpSec1 transport mix with 0.33 mg/mL Chl thylakoids, 0.83 mg/mL Chl equivalent SE or 1.5 μg of recombinant cpSecA1, and 5 mM ATP on ice, and incubate for 10 min. A typical transport reaction mixture here is 72 μL, up from 60 μL due to the SE addition.
- If recombinant cpSecA1 will be used instead of SE, adjust purified cpSecA1 with an equal volume of 2xIB, then dilute to a convenient concentration to add to the transport reactions (e.g., 0.75 µg/µL).
Note: For long term storage, cpSecA1 is stored on ice in a controlled, refrigerated room after purification as freezing abolishes activity.
- If recombinant cpSecA1 will be used instead of SE, adjust purified cpSecA1 with an equal volume of 2xIB, then dilute to a convenient concentration to add to the transport reactions (e.g., 0.75 µg/µL).
- Initiate transport by introducing 1:10 volume by volume substrate protein to the transport mix. If substrate protein is not prepared in 1xIB, prepare a 1:1 dilution with 2xIB and add 1:10 volume by volume of the diluted protein to the transport mix.
- Incubate the reaction at room temperature for 10 min under 80 – 100 µE/m2s PAR. Again, longer times may be necessary for kinetics or certain substrates.
- After light treatment, dilute 8-fold with ice-cold 1xIB and centrifuge reactions at 3200 g in microcentrifuge for 5 min at 4 °C.
- Treat with thermolysin and quench with EDTA as above in Steps 5.5 through 5.7.
- Centrifuge at 3,200 g in microcentrifuge for 5 min at 4 °C and resuspend the pellet in an appropriate volume of Laemmli Sample Buffer supplemented with 10 mM EDTA. Place in boiling water bath for 10 min prior to loading on SDS-PAGE gel.
7. Insertion through the cpSRP Pathway
Note: The cpSRP-mediated integration of light harvesting complex proteins (LHCP) seen in Figure 3 requires cpSRP54, cpSRP43, and cpFtsY16. These components are supplied to the transport reaction through concentrated stromal extract, as described for the cpSec1 transport protocol.
- Prepare the cpSRP transport mix with 0.33 mg/mL Chl thylakoids, 0.83 mg/mL Chl equivalent SE, and 5 mM ATP on ice and incubate for 10 min.
- Initiate transport by introducing 1:10 volume by volume substrate protein to the transport mix. If substrate protein is not prepared in 1xIB, prepare a 1:1 dilution with 2xIB and add 1:10 volume by volume of the diluted protein to the transport mix.
- Incubate reaction at room temperature for 10 min in 80 – 100 µE/m2s PAR.
- After light treatment, dilute 8-fold with ice-cold 1xIB and centrifuge reactions at 3,200 g in microcentrifuge for 5 min at 4 °C. Discard supernatant and resuspend the pellet in 120 µL of 1xIB.
- Treat with thermolysin as above in sections 5.5 through 5.7. Thermolysin digestion is necessary here to assess successful membrane integration. Wash recovered thylakoids in 120 μL of 5 mM EDTA in 1xIB and transfer to a new tube.
- Centrifuge at 3,200 g in microcentrifuge for 5 min at 4 °C and resuspend the pellet again in an appropriate volume of 2x Laemmli buffer supplemented with 10 mM EDTA. Place in boiling water bath for 10 min prior to analysis by SDS-PAGE.
Note: Integration of mature LHCP (mLHCP) is assessed by the appearance of a protease-protected degradation product (mLHCP-D) approximately 1.5-2 kDa smaller than the uncleaved mLHCP17.
Representative Results
To gauge amount of substrate successfully transported, it is useful to include one or more "percent input" lanes. For the data presented below, 10% of the final transport reaction without thylakoids was used. This "percent input" also helps to visualize the size of the precursor substrate. The percentage represents a known, defined amount of substrate with which to compare transported substrate against and can be scaled up or down as necessary using initially prepared protein. Additionally, it is advisable to load less than 4 µg of Chl equivalents in a single lane on 0.75 mm polyacrylamide gels to avoid band warping and smearing. All substrates below were prepared and radiolabeled using in vitro translation kits.
Transport of iOE17 through cpTat pathway
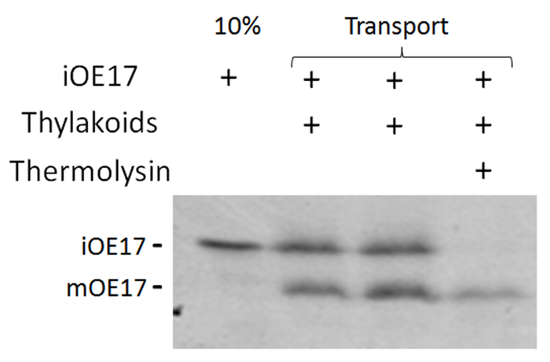
Figure 1. Transport of iOE17through the cpTat pathway. Fluorograph of [3H]-iOE17 transport assay and thermolysin treatment of non-transported substrate. Please click here to view a larger version of this figure.
Successful cpTat transport of most Tat substrates can be detected by a size shift upon cleavage of the N-terminal signal peptide3. In substrates where no cleavable signal peptide exists18,19, protease treatment is required to reveal the substrate that crossed the membrane, thereby becoming inaccessible to digestion by the protease. In Figure 1, transport of iOE17 results in a size shift of approximately 2-3 kDa between the introduced substrate and the mature processed form. Non-transported substrate is degraded through protease treatment (lane 4).
Transport of prOE33 through cpSec1 pathway
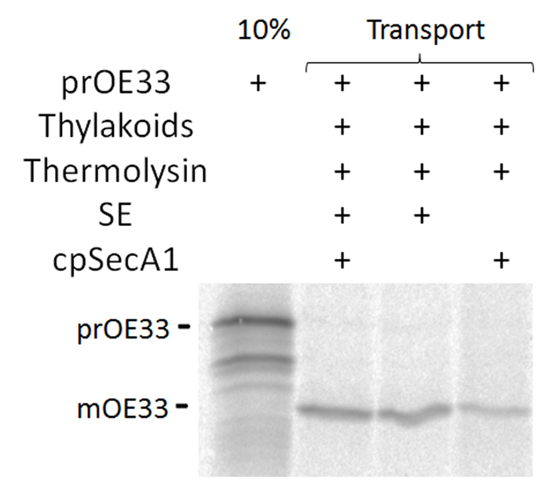
Figure 2. Transport of prOE33 through cpSec1 pathway. Autoradiograph of [35S]-prOE33 transport assay using SE, purified cpSecA1, or both simultaneously. Please click here to view a larger version of this figure.
Transport through the cpSec1 pathway (Figure 2) can be evaluated through a size shift in the mature substrate if the substrate contains a cleavable thylakoid targeting signal, as well as protease protection of transported substrate20.
Integration of prLHCP through cpSRP pathway
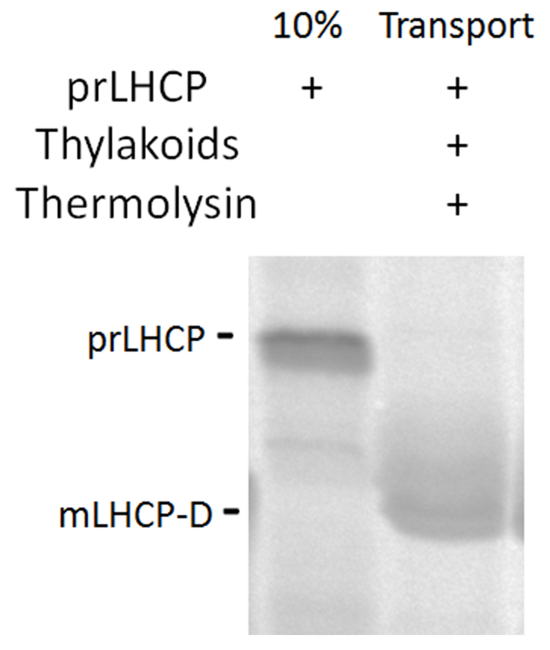
Figure 3. Integration of prLHCP through cpSRP pathway. Autoradiograph of [35S]-prLHCP and digestion to the product mLHCP-D after insertion into the thylakoid membrane. Please click here to view a larger version of this figure.
Insertion of prLHCP into the thylakoid membrane via the cpSRP pathway can be evaluated through thermolysin digestion. A size shift of approximately 1.5-2 kDa is indicative of successful membrane insertion as the membrane protects the uncleaved mature protein from complete proteolysis15. In Figure 3, the protease-protected product mLHCP-D is clearly visible.
Discussion
Chloroplast and Thylakoid isolation
Excessive breakage can result in poor chloroplast isolation and thus poor thylakoid yield after separation in the gradient. It is best to homogenize the harvested tissue gently by ensuring that all material is submerged before blending and pulsing in 15 s cycles until fully homogenized. If necessary, use multiple shorter rounds of blending with less tissue in each round.
Refrigerating all materials that come into contact with harvested tissue helps isolated chloroplasts to retain activity up to 2 hours. It is important to keep the chloroplasts on ice in the dark after isolation as well.
It is crucial to include magnesium in buffers contacting isolated thylakoids. Not doing so results in granal destacking and critically affects the generation of proton motive force upon illumination21. Thylakoids have been used in transport reactions up to 2 hours after isolation when kept on ice and in the dark.
Optimizing Transport Reactions
Isolated thylakoids settle rapidly and can result in unequal Chl equivalents between reactions. As such, it is important to mix the thylakoids thoroughly prior to use, especially when setting up a large number of samples.
The Tat Pathway
Transport efficiency through the Tat pathway can be reduced upon excessive washing of the thylakoids since Tha4 (TatA) can be partially extracted from the membrane22. In such cases, it is helpful to reduce thylakoid wash steps prior to the transport reaction. Additionally, longer illumination times can improve detection where substrates are poorly transported under typical conditions.
The cpSec1 Pathway
When assaying the cpSec1 pathway, the amount of cpSecA1 in concentrated SE should support transport, but that transport may be weak. Efficient transport in isolated thylakoids may benefit from the addition of purified cpSecA1 for certain proteins, though stromal chaperones in SE may also help increase the efficiency of transport15. Further, preparations of cpSecA1 protein should not be frozen prior to usage in transport, as this reduces transport activity. Similarly, frozen SE is less efficacious than freshly prepared extract. As in Tat transport, longer incubation periods in the transport mix can help with difficult substrates.
The cpSRP Pathway
While reconstitution of cpSRP transport using individual components has been performed23, it is convenient to supply cpSRP43, cpSRP54, and cpFtsY with SE. Thermolysin resistance is the most stringent criteria for LHCP integration16,17, but alkaline extraction may be performed as well17. While precursors can often be frozen and stored at -80 °C for many transport reactions, prLHCP prepared by in vitro synthesis should be used for transport immediately after synthesis without freezing.
Characterization of a Novel Substrate's Targeting Pathway
In cases where the translocation pathway taken by a novel substrate is unknown, it is advisable to first investigate possible signal peptides in silico using the amino acid sequence of the precursor or intermediate form. The cpTat pathway typically requires a specific twin arginine consensus motif in the signal peptide and no ATP for successful transport under PAR3. Unlike the Tat pathway, the cpSec1 pathway requires ATP and the cpSecA1 protein. Failed transport in conditions lacking these components suggests the cpSec1 pathway20. The cpSRP pathway requires the signal recognition particle found in the SE. Failed transport using purified cpSecA1 and ATP, but a lack of twin arginine consensus motif in the signal peptide, suggests the cpSRP pathway23.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This manuscript was prepared with funding by the Division of Chemical Sciences, Geosciences, and Biosciences, 408 Office of Basic Energy Sciences of the US Department of Energy through Grant DE-SC0017035
Materials
| Pisum sativum seeds | Seedway LLC, Hall, NY | 8686 – Little Marvel | |
| Miracloth | Calbiochem, Gibbstown, NJ | 475855-1 | |
| 80% Acetone | Sigma, Saint Louis, MO | 67-64-1 | |
| Blender with sharpened blades | Waring Commercial | BB155S | |
| Polytron 10-35 | Fischer Sci | 13-874-617 | |
| Percoll | Sigma, Saint Louis, MO | GE17-0891-01 | |
| Beckman J2-MC with JA 20 rotor | Beckman-Coulter | 8043-30-1180 | |
| Sorvall RC-5B with HB-4 rotor | Sorvall | 8327-30-1016 | |
| 100 mM dithiothreitol (DTT) in 1xIB | Sigma, Saint Louis, MO | 12/3/83 | Can be frozen in aliquots for future use |
| 200 mM MgATP in 1xIB | Sigma, Saint Louis, MO | 74804-12-9 | Can be frozen in aliquots for future use |
| Thermolysin in 1xIB (2mg/mL) | Sigma, Saint Louis, MO | 9073-78-3 | Can be frozen in aliquots for future use |
| HEPES | Sigma, Saint Louis, MO | H3375 | |
| K-Tricine | Sigma, Saint Louis, MO | T0377 | |
| Sorbitol | Sigma, Saint Louis, MO | 50-70-4 | |
| Magnesium Chloride | Sigma, Saint Louis, MO | 7791-18-6 | |
| Manganese Chloride | Sigma, Saint Louis, MO | 13446-34-9 | |
| EDTA | Sigma, Saint Louis, MO | 60-00-4 | |
| BSA | Sigma, Saint Louis, MO | 9048-46-8 | |
| Tris | Sigma, Saint Louis, MO | 77-86-1 | |
| SDS | Sigma, Saint Louis, MO | 151-21-3 | |
| Glycerol | Sigma, Saint Louis, MO | 56-81-5 | |
| Bromophenol Blue | Sigma, Saint Louis, MO | 115-39-9 | |
| B-Mercaptoethanol | Sigma, Saint Louis, MO | 60-24-2 |
References
- Ellis, R. Chloroplast protein synthesis: principles and problems. Sub-cellular biochemistry. 9, 237 (1983).
- Li, H. -. m., Chiu, C. -. C. Protein transport into chloroplasts. Annual review of plant biology. 61, (2010).
- Cline, K., Ettinger, W., Theg, S. M. Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. Journal of Biological Chemistry. 267 (4), 2688-2696 (1992).
- Skalitzky, C. A., et al. Plastids contain a second sec translocase system with essential functions. Plant physiology. 155 (1), 354-369 (2011).
- Dabney-Smith, C., Storm, A. . Plastid Biology. , 271-289 (2014).
- Kim, S. J., Jansson, S., Hoffman, N. E., Robinson, C., Mant, A. Distinct "assisted" and "spontaneous" mechanisms for the insertion of polytopic chlorophyll-binding proteins into the thylakoid membrane. Journal of Biological Chemistry. 274 (8), 4715-4721 (1999).
- Emanuelsson, O., Nielsen, H., Von Heijne, G. C. h. l. o. r. o. P. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Science. 8 (5), 978-984 (1999).
- Emanuelsson, O., Brunak, S., Von Heijne, G., Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nature protocols. 2 (4), 953 (2007).
- Ling, Q., Jarvis, R. Analysis of protein import into chloroplasts isolated from stressed plants. Journal of Visualized Experiments. (117), e54717 (2016).
- Lo, S. M., Theg, S. M. . Photosynthesis Research Protocols. , 139-157 (2011).
- Vernon, L. P. Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Analytical Chemistry. 32 (9), 1144-1150 (1960).
- Knott, T. G., Robinson, C. The secA inhibitor, azide, reversibly blocks the translocation of a subset of proteins across the chloroplast thylakoid membrane. Journal of Biological Chemistry. 269 (11), 7843-7846 (1994).
- Yuan, J., Henry, R., McCaffery, M., Cline, K. SecA homolog in protein transport within chloroplasts: evidence for endosymbiont-derived sorting. Science. 266 (5186), 796-798 (1994).
- Nohara, T., Nakai, M., Goto, A., Endo, T. Isolation and characterization of the cDNA for pea chloroplast SecA Evolutionary conservation of the bacterial-type SecA-dependent protein transport within chloroplasts. FEBS letters. 364 (3), 305-308 (1995).
- Endow, J. K., Singhal, R., Fernandez, D. E., Inoue, K. Chaperone-assisted post-translational transport of plastidic type I signal peptidase 1. Journal of Biological Chemistry. 290 (48), 28778-28791 (2015).
- Luirink, J., Sinning, I. SRP-mediated protein targeting: structure and function revisited. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1694 (1-3), 17-35 (2004).
- Yuan, J., Henry, R., Cline, K. Stromal factor plays an essential role in protein integration into thylakoids that cannot be replaced by unfolding or by heat shock protein. Hsp70. Proceedings of the National Academy of Sciences. 90 (18), 8552-8556 (1993).
- Tjalsma, H., van Dijl, J. M. Proteomics-based consensus prediction of protein retention in a bacterial membrane. Proteomics. 5 (17), 4472-4482 (2005).
- Widdick, D. A., Eijlander, R. T., van Dijl, J. M., Kuipers, O. P., Palmer, T. A Facile Reporter System for the Experimental Identification of Twin-Arginine Translocation (Tat) Signal Peptides from All Kingdoms of Life. Journal of Molecular Biology. 375 (3), 595-603 (2008).
- Yuan, J., Cline, K. Plastocyanin and the 33-kDa subunit of the oxygen-evolving complex are transported into thylakoids with similar requirements as predicted from pathway specificity. Journal of Biological Chemistry. 269 (28), 18463-18467 (1994).
- Kirchhoff, H., Borinski, M., Lenhert, S., Chi, L., Büchel, C. Transversal and lateral exciton energy transfer in grana thylakoids of spinach. Biochemistry. 43 (45), 14508-14516 (2004).
- Frielingsdorf, S., Jakob, M., Klösgen, R. B. A stromal pool of TatA promotes Tat-dependent protein transport across the thylakoid membrane. Journal of Biological Chemistry. 283 (49), 33838-33845 (2008).
- Tu, C. -. J., Schuenemann, D., Hoffman, N. E. Chloroplast FtsY, chloroplast signal recognition particle, and GTP are required to reconstitute the soluble phase of light-harvesting chlorophyll protein transport into thylakoid membranes. Journal of Biological Chemistry. 274 (38), 27219-27224 (1999).

