Using Microarrays to Interrogate Microenvironmental Impact on Cellular Phenotypes in Cancer
Summary
The purpose of the method presented here is to show how microenvironment microarrays (MEMA) can be fabricated and used to interrogate the impact of thousands of simple combinatorial microenvironments on the phenotype of cultured cells.
Abstract
Understanding the impact of the microenvironment on the phenotype of cells is a difficult problem due to the complex mixture of both soluble growth factors and matrix-associated proteins in the microenvironment in vivo. Furthermore, readily available reagents for the modeling of microenvironments in vitro typically utilize complex mixtures of proteins that are incompletely defined and suffer from batch to batch variability. The microenvironment microarray (MEMA) platform allows for the assessment of thousands of simple combinations of microenvironment proteins for their impact on cellular phenotypes in a single assay. The MEMAs are prepared in well plates, which allows the addition of individual ligands to separate wells containing arrayed extracellular matrix (ECM) proteins. The combination of the soluble ligand with each printed ECM forms a unique combination. A typical MEMA assay contains greater than 2,500 unique combinatorial microenvironments that cells are exposed to in a single assay. As a test case, the breast cancer cell line MCF7 was plated on the MEMA platform. Analysis of this assay identified factors that both enhance and inhibit the growth and proliferation of these cells. The MEMA platform is highly flexible and can be extended for use with other biological questions beyond cancer research.
Introduction
Culturing of cancer cell lines on plastic in two-dimensional (2D) monolayers remains one of the major workhorses for cancer researchers. However, the microenvironment is increasingly being recognized for its ability to impact cellular phenotypes. In cancer, the tumor microenvironment is known to influence multiple cellular behaviors, including growth, survival, invasion, and response to therapy1,2. Traditional monolayer cell cultures typically lack microenvironment influences, which has led to the development of more complex three-dimensional (3D) assays to grow cells, including commercially available purified basement membrane extracts. However, these purified matrices are typically complicated to use and suffer from technical problems such as batch variability3 and complex compositions3. As a result, it can be difficult to assign function to specific proteins that may be impacting cellular phenotypes3.
To address these limitations, we have developed the microenvironment microarray (MEMA) technology, which reduces the microenvironment down to simple combinations of extracellular matrix (ECM) and soluble growth factor proteins4,5. The MEMA platform enables identification of dominant microenvironmental factors that impact the behavior of cells. By using an array format, thousands of combinations of microenvironment factors can be assayed in a single experiment. The MEMA described here interrogates ~2,500 different unique microenvironment conditions. ECM proteins printed into well plates form growth pads upon which cells can be cultured. Soluble ligands are added to individual wells, creating unique combinatorial microenvironments (ECM + ligand) on each different spot to which the cells are exposed. Cells are cultured for several days, then fixed, stained, and imaged to assess cellular phenotypes as a result of exposure to these specific microenvironment combinations. Since the microenvironments are simple combinations, it is straightforward to identify proteins that drive major phenotypic changes in cells. MEMAs have been used successfully to identify factors that influence multiple cellular phenotypes, including those that drive cell fate decisions and response to therapy4,5,6,7. These responses can be validated in simple 2D experiments and can then be assessed under conditions that more fully recapitulate the complexity of the tumor microenvironment. The MEMA platform is highly adaptable to a variety of cell types and endpoints, provided that good phenotypic biomarkers are available.
Protocol
NOTE: An overview of the entire MEMA process, including estimated time, is outlined in the flow diagram shown in Figure 1. This protocol details the fabrication of MEMAs in 8-well plates. The protocol may be adapted for other plates or slides.
1. Preparation of Protein, Diluent, and Staining Buffers
- Equilibrate vials of ECMs, ligands, and cytokines to room temperature (RT) and briefly centrifuge. Add the appropriate volume of the appropriate RT buffer as indicated on the product data sheet. Follow manufacturer’s recommendation for stock concentrations.
NOTE: A full list of the ligands and ECMs with their stock and final concentrations are provided in Table 1 and Table 2. Both ligands and ECMs are typically used at the highest concentration of the range recommended by the manufacturer that elicits a biological effect in standard 2 day culture assays. Handle proteins gently and in biosafety cabinets under laminar flow to avoid contamination. - Incubate vials with gentle rocking at RT for 1 h. Do not vortex proteins as this can cause them to denature.
- Aliquot proteins for long term storage so that all aliquots are single use only to avoid degradation with repeated freeze/thaw cycles. Store lyophilized proteins at -80 °C (unless otherwise specified) until needed. Take care to collect all metadata for future reference, such as: (i) protein name, (ii) date prepared, (iii) lot/batch number, (iv) supplier, (v) catalog number, (vi) concentration, (vii) volume, and (viii) preparer.
- Prepare diluent buffer containing 20% (v/v) glycerol, 10 mM EDTA, 200 mM Tris-HCl, pH 7.2, and filter sterilize. Keep this buffer sterile and store at RT.
- Prepare staining buffer containing 2% (w/v) BSA, 1 mM MgCl2, and 0.02% NaN3 in phosphate-buffered saline (PBS). Filter and store at 4 °C.
2. Preparation of an ECM Source Plate
- Remove aliquoted stocks of ECM proteins to be printed and thaw on ice. Record all lot numbers for metadata tracking.
- Flick tubes of thawed proteins gently to ensure proper resuspension and spin down in a centrifuge.
- Make ECM print mixtures (EPMs) and a fluorescent fiducial to be used by a liquid handling robot that will create the randomized 384-well source plates.
NOTE: The 384-well source plates will be used by a touch pin array printer to create the printed arrays in 8 well plates.- Label 1.5 mL microcentrifuge tubes for each EPM and the fiducial.
- Prepare each EPM by combining 125 µL of diluent buffer (see step 1.4) with the appropriate volume of ECM stock and bring the mixture up to a total volume of 250 µL with PBS. The final concentrations in each EPM tube will be 1x ECM protein, 5 mM EDTA, 10% glycerol, and 100 mM Tris.
- Prepare a fluorescent fiducial by dissolving it in the appropriate buffer specified by the manufacturer and transfer 250 µL to a labelled fiducial tube.
3. Creation of the Source Plate Using a Liquid Handler
- Design a 384-well plate layout that randomizes the positions of the ECMs and is optimized for the array printer pin head being used. Design the placement of the fiducial so that it will be printed in the row 1, column 1 position of each well to assist in array orientation.
NOTE: A total of 14−15 replicates of each ECM are used to ensure robust data. Include additional replicates of collagen or another ECM that yields robust attachment for assessment of uniformity of binding. The layout may need to utilize multiple 384-well plates depending on the number of ECMs of interest. - Transfer EPM tubes to a liquid handler, keeping tubes at 4 °C either with a cooled tube rack or by using a liquid handling robot located in a cold room.
- Using the liquid handler’s software, run a program to transfer 15 µL of each EPM and the fiducial to the predesignated wells within the 384-well source plate(s).
- Pipet PBS into any unused wells to increase humidity and guard against desiccation during the printing process.
NOTE: See Figure 2 for an example of a 384-well source plate set that is optimized for a 4 x 7 pin head and includes a collagen I block and PBS. - Seal plate(s) and keep at 4 °C until ready to print.
4. Printing MEMAs Using an Array Printing Robot
NOTE: The following part of the protocol specifically describes the preparation and use of MEMA to investigate the impact of different microenvironment proteins on the growth and proliferation of MCF7 cells. However, the protocol can easily be adapted to use different ligands, ECMs, and cells to study other cell lines and endpoints of interest.
- Using a touch pin printer, print EPMs and fiducial spots into 8 well plates. Print multiple replicates of each ECM condition to ensure reproducibility.
NOTE: Other plate formats or slides can be used for printing, but buffer optimization may be required to achieve optimal spot formation.- Print the ECMs for the MEMA using 350 µm diameter pins arranged in a 4 x 7 print head configuration. Print the arrays in the 8-well plates as 20 columns by 35 rows, for a total of ~700 spots. Larger arrays are possible in these plates but come with a trade-off of increased edge effects in both cell binding and staining.
- After printing, store plates in a desiccator for a minimum of 3 days prior to use.
5. Creation of Ligand Treatment Plates
- Design a 96-well plate layout including ligands of interest. To facilitate treatment of many MEMA plates at once, design this plate with spacing that allows for the use of a multi-channel pipet with 4 spaced tips to transfer liquids between the wells of 8-well MEMAs and a 96-well plate.
NOTE: In this protocol, the full set of ligands listed in Table 2 are utilized. - Thaw ligands on ice. Briefly flick and spin down each tube.
- Dilute ligands to a 200x working stock using the manufacturer’s recommended buffer (typically PBS).
- Pipet 10 µL of each 200x ligand stock into the corresponding well within the 96-well plate.
- Seal and store plates at -20 °C.
NOTE: Make ligand treatment plates in batches, capturing all metadata for downstream analysis.
6. Culturing Cells on MEMAs
- Block MEMAs for 20 min with 2 mL per well of non-fouling blocking buffer containing 1% non-fouling blocking agent (Table of Materials) in double-distilled water (ddH2O).
- Aspirate blocking buffer and triple rinse wells with PBS. To prevent desiccation, leave final volume of PBS in wells until ready for cell plating.
NOTE: It is extremely helpful to have two bench workers for cell culture steps on MEMAs. One bench worker can perform aspiration steps, while the second performs addition steps. It is recommended to use a 1 mL multichannel pipet with tips spaced to match the 8-well plate for pipetting and a Y-splitter with two Pasteur pipettes to aspirate multiple wells at once. - Seed 2 x 105 MCF7 cells per well in 2 mL of Dulbecco’s modified Eagle’s medium (DMEM) medium containing 10% fetal bovine serum (FBS).
NOTE: Prior to a full MEMA experiment, perform a cell titration experiment to optimize cell numbers such that MEMA spots have high cell numbers (but are not confluent) at the end of the desired experimental duration. - After 2−18 h of adhesion, aspirate medium and replace with 2 mL of reduced-growth medium (DMEM with 0.1% FBS).
NOTE: Reduced serum (e.g., 0.1% FBS) or growth factor-depleted conditions can be used at this time to isolate the stimulatory impact of specific ligands. - Thaw a ligand treatment plate on ice. Centrifuge thawed plate at 200 x g for 1 min.
- Transfer 200 µL of medium from each well in the culture plate to the appropriate well in the treatment plate. Pipet up and down to mix ligand volume with medium and transfer this mixture back to the appropriate well in the MEMA plate.
- Lightly rock by hand and return MEMA plates to the incubator. Culture for the duration of the experiment in the presence of the ligand/ECM combination at 37 °C and 5% CO2.
NOTE: A typical MEMA experiment runs for 72 h; longer duration experiments may require replacement of medium and re-treatment with ligand. - Pulse MEMA wells at 71 h with 100x 5-ethynyl-2’-deoxyuridine (EdU) for a final concentration of 10 µM. Incubate in experimental conditions with EdU for 1 h at 37 °C and 5% CO2.
NOTE: Other live cell treatments may also be used at this time.
7. Fixing and Staining MEMAs
- After 72 h and any live cell treatments, aspirate wells. Fix MEMAs in 2 mL per well of 2% paraformaldehyde (PFA) for 15 min at RT.
- Aspirate PFA. Permeabilize with 2 mL per well of 0.1% nonionic surfactant for 15 min.
- Aspirate the nonionic surfactant and wash with 2 mL per well of PBS. Aspirate PBS. Wash with 2 mL of PBS with 0.05% polysorbate 20 (PBS-T).
NOTE: The MEMA surface is hydrophobic, and failure to wash with PBS-T before stain and antibody incubation will result in the formation of voids in wells during incubation steps and give rise to staining artifacts. - Aspirate PBS-T. Add EdU detection reaction reagents. Incubate for 1 h at RT, rocking and protected from light. After 1 h incubation, quench reaction with the provided commercial quench buffer.
NOTE: EdU detection and staining/antibody steps may be performed in 1.5 mL per well to reduce cost. - Aspirate the quench buffer and wash with PBS-T prior to incubating with stains or antibodies.
- Incubate MEMA wells with antibodies against histone H3K9me3 (1:1,000) and fibrillarin (1:400) in staining buffer containing 2% (w/v) bovine serum albumin (BSA), 1 mM MgCl2 and 0.02% NaN3 overnight at 4 °C.
NOTE: Perform antibody titrations to determine optimal concentrations prior to using them on a full MEMA set. - Following primary antibody or stain incubation, wash wells 2x with PBS and once with PBS-T.
- Add secondary antibodies (donkey anti-mouse IgG and donkey anti-rabbit IgG, both 1:300) and 0.5 µg/mL 4′ 6‐diamidino‐2‐phenylindole (DAPI). Incubate for 1 h at RT in the dark.
- Wash wells 2x with 2 mL per well of PBS, leaving them in the final 2 mL PBS.
- Proceed to imaging or store stained MEMAs for later imaging in PBS at 4 °C protected from light.
8. Imaging of MEMAs
- Image MEMA on an automated imaging system with appropriate fluorescent detection channels.
- Output resulting image data to an image management system. Segment cells and calculate intensity levels using CellProfiler8.
9. Data Analysis
NOTE: Data analysis consists of normalization, variation correction, and summarization of the raw CellProfiler derived data. In this instance, the R-environment with custom code is used to perform all the steps. However, any statistical environment or software program can be utilized to perform the equivalent actions. An example of the open source custom code for the R environment for analysis is available at: https://www.synapse.org/#!Synapse:syn2862345/wiki/72486.
- Preprocess and normalize the segmented image data.
- Determine spot cell count using the DAPI stained nuclei.
- Auto-gate EdU intensity to label cells as EdU+. Measure proliferation using the proportion of EdU+ cells in each spot.
- Median summarize cytoplasmic stains and nuclear morphology measurements on the spot level.
- Perform removal of unwanted variation (RUV) normalization on the data to improve data quality9.
NOTE: This approach is applied to each intensity and morphology signal independently as a matrix with arrays using the rows and spots as the columns as described previously9. - Apply bivariate loess normalization to the RUV normalized residuals using the array row and array column as the independent variables to correct for spatial or intensity related effects.
- Once normalization is completed, median summarize the replicates for each microenvironment condition for reporting and further analysis.
Representative Results
To simplify microenvironmental impacts on cell growth and proliferation and to identify conditions that promoted or inhibited cell growth and proliferation, the breast cancer cell line MCF7 was seeded on a set of eight 8-well MEMAs as described in the protocol. This assay exposed the cells to 48 different ECMs and 57 different ligands, for a total of 2736 combinatorial microenvironmental conditions. After 71 h in culture, cells were pulsed with EdU, fixed, permeablized, and stained with DAPI, the reaction for EdU detection, an anti-fibrillin antibody, and an anti-H3K9me3 antibody. Cells were imaged on a high content microscope. The images were uploaded to an Omero server10, segmented using CellProfiler8, and normalized and analyzed in R9. The results described below focus on the DAPI and EdU signals.
The image analysis platform of MEMAs yields some results similar to those available from flow cytometry approaches, such as DNA content plots showing 2N and 4N fractions for cells treated with a given ligand (Figure 3A), based on the DAPI intensity and area. These plots provide evidence for conditions that promote active cell cycling versus as indicated by clear bimodal peaks corresponding to cells in G1 or G2 phases vs. growth arrested cells, which would show changes in the peaks compared to control conditions. We use the cell number and staining intensity data to summarize the data, where the impact of the microenvironment (ligands on one axis, ECM on the second axis) on both cell number (Figure 3B) and EdU incorporation (Figure 3C) can be more easily seen as changes in heatmap color and intensity. As seen from these plots, many of the effects are ligand-driven, as the ECM condition did not strongly impact cell number or EdU positivity. Nidogen-1 is a clear exception, as the presence of this ECM molecule inhibits cell binding and growth of MCF7. Ligands such as FGF6 and NRG1α (NRG1.1 on plots) enhance cell number and have high rates of EdU incorporation, while ligands such as AREG and NRG1-smdf (NRG1.10 on plots) inhibit cell binding and/or growth of cells. These findings are supported by the images of the cells growing on the spots, where a clear difference in cell number and EdU positivity is evident (see example in Figure 3D).
Since the MEMA platform is a newer technology, results were validated in separate assays. MCF7 cells were seeded into 24-well plates coated with collagen I in DMEM medium with 10% FBS. After 18 h, media were exchanged for reduced growth medium (DMEM with 0.1% FBS) and cells were treated with NRG1α, FGF6, or AREG and cultured for 72 h. EdU was added 1 h prior to fixation. Cells were stained with DAPI and for EdU incorporation, imaged, segmented, and analyzed. Similar to the results obtained from the MEMA platform, FGF6 and NRG1α both gave rise to higher cell numbers (Figure 4A) and EdU incorporation rates (Figure 4B) compared to AREG treated cells, validating our observations in the original MEMA experiments.
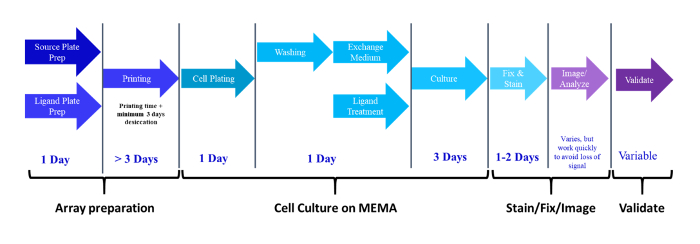
Figure 1: Flow chart showing the workflow and timeline for the different phases of a typical MEMA experiment. Once the MEMAs are printed, they can be stored at room temperature desiccated for several months prior to use. Typically, the experimental phase lasts 3−4 days, but some slow growing primary cells have been cultured on MEMAs for up to 2 weeks. Please click here to view a larger version of this figure.
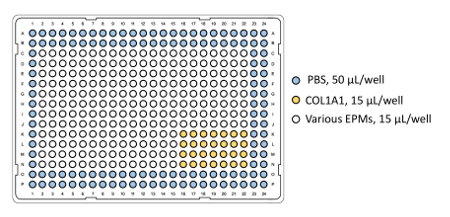
Figure 2: ECM source plate layout for array printing. The collagen block is printed onto MEMA as a grid, which provides a highly repetitive set of conditions that allow for more robust normalization between wells. The PBS-filled wells provide humidity to aid in prevention of evaporation during the printing process. Please click here to view a larger version of this figure.
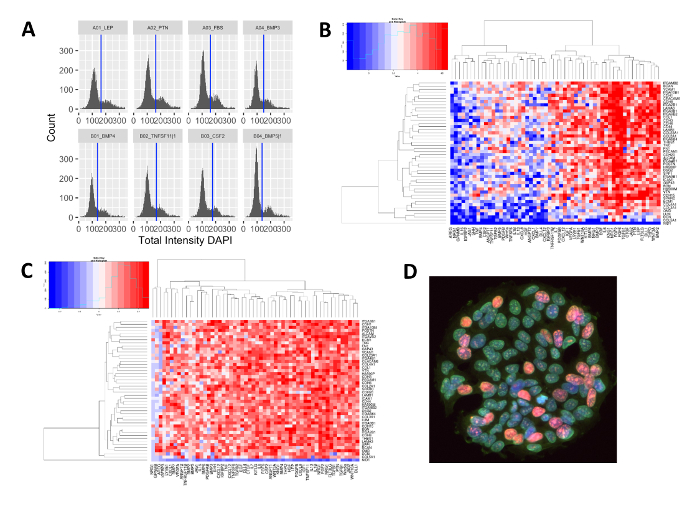
Figure 3: Examples of data generated from a typical MEMA experiment. (A) Cell cycle profiles of binned DAPI intensity values versus cell counts from one 8-well plate treated with different ligands, showing biphasic DAPI intensity staining indicating cells in G1 versus G2 cell cycle phase. (B) Heatmap showing normalized spot cell counts clustered by similarity using hierarchical clustering. Red indicates higher cell number, and blue is lower cell number. Ligands are on the x-axis, ECMs are on the y-axis. (C) Heatmap showing normalized EdU incorporation, with red indicating higher and blue indicating lower EdU incorporation. Ligands are on the x-axis, ECMs are on the y-axis. (D) Example of MCF7 cells growing on a MEMA spot treated with NRG1-α showing high rates of EdU incorporation (pink nuclei). Green stain is cell mask and blue is DAPI. Please click here to view a larger version of this figure.
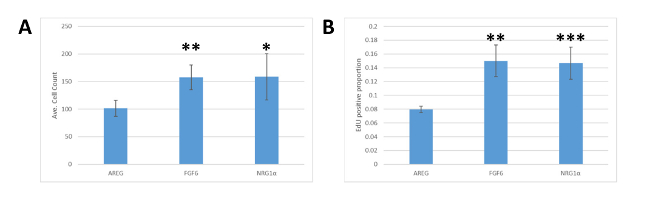
Figure 4: Validation of MEMA results in cell culture. (A) Quantification of cell number resulting from treatment of MCF7 with different ligands. Equivalent numbers of MCF7 cells were plated into multiwall plates then treated with either AREG, FGF6, or NRG1α. Wells treated with AREG had significantly fewer cells than those treated with FGF6 (** indicating student’s t-test p-values less than 0.01) or NRG1α (* indicates a p-value of 0.05) at 72 h post ligand treatment. (B) Quantification of the level of EdU incorporation in MCF7 due to treatment with different ligands, as in panel A. AREG treatment results in a significantly lower proportion of cells incorporating EdU than cells treated with FGF6 (**, p < 0.01) or NRG1α (***, p = 0.01). Error bars represent standard deviation. Please click here to view a larger version of this figure.
| Protein Name | Uniprot ID | Stock Concentration (µg/mL) |
Final Concentration (µg/mL) |
| ANGPT1|1 | Q15389|1 | 100 | 0.04 |
| ANGPT2|1 | O15123|1 | 100 | 0.2 |
| AREG | P15514 | 100 | 0.02 |
| BMP2 | P12643 | 100 | 0.1 |
| BMP3 | P12645 | 1000 | 0.1 |
| BMP4 | P12644 | 100 | 0.1 |
| BMP5|1 | P22003|1 | 100 | 0.1 |
| BMP6 | P22004 | 100 | 0.1 |
| BMP7 | P18075 | 100 | 0.1 |
| CSF2 | P04141 | 100 | 0.02 |
| CTGF|1 | P29279|1 | 100 | 0.05 |
| CXCL12|Alpha | P48061|2 | 100 | 0.01 |
| CXCL12|Beta | P48061|1 | 100 | 0.03 |
| CXCL1 | P09341 | 100 | 0.004 |
| CXCL8|1 | P10145|1 | 100 | 0.3 |
| DLL1|1 | O00548|1 | 500 | 0.5 |
| DLL4 | Q9NR61 | 200 | 0.6 |
| EGF|1 | P01133|1 | 500 | 0.01 |
| FASLG|1 | P48023|1 | 10 | 0.02 |
| FGF2|3 | P09038|2 | 100 | 0.01 |
| FGF6 | P10767 | 100 | 0.01 |
| FLT3LG|1 | P49771|1 | 50 | 0.001 |
| GPNMB|1 | Q14956|1 | 100 | 0.5 |
| HGF|1 | P14210|1 | 50 | 0.04 |
| IGF1|1 | P05019|1 | 200 | 0.01 |
| IGFBP2 | P18065 | 100 | 0.05 |
| IGFBP3|1 | P17936|1 | 100 | 0.1 |
| IL13 | P35225 | 100 | 0.01 |
| IL15|IL15S48AA | P40933|1 | 50 | 0.01 |
| IL1B | P01584 | 25 | 0.001 |
| IL6 | P05231 | 100 | 0.01 |
| IL7|1 | P13232|1 | 100 | 0.01 |
| JAG1|1 | P78504|1 | 200 | 0.5 |
| JAG2|Long | Q9Y219|1 | 100 | 0.5 |
| KITLG|1 | P21583|1 | 100 | 0.005 |
| KNG1|HMW | P01042|1 | 100 | 0.2 |
| LEP | P41159 | 1000 | 0.002 |
| LYVE1 | Q9Y5Y7 | 100 | 0.05 |
| NRG1|10 | Q02297|10 | 100 | 0.01 |
| NRG1|1 | Q02297|1 | 100 | 0.05 |
| NRG1|6 | Q02297|6 | 100 | 0.01 |
| PDGFAB | go1990265 | 100 | 0.05 |
| PDGFB|1 | P01127|1 | 100 | 0.05 |
| PTN | P21246 | 100 | 0.5 |
| SHH | Q15465 | 100 | 0.5 |
| TGFB1||Cterminus | P01137|Cterminus | 20 | 0.01 |
| TGFB1||LAP | P01137|LAP | 100 | 0.15 |
| TGFB2|A | P61812|1 | 20 | 0.01 |
| THPO|1 | P40225|1 | 50 | 0.002 |
| TNFRSF11B | O00300 | 100 | 0.02 |
| TNFSF11|1 | O14788|1 | 100 | 0.01 |
| TNF | P01375 | 100 | 0.01 |
| VEGFA|VEGF206 | P15692|1 | 100 | 0.01 |
| WNT10A | Q9GZT5 | 100 | 0.1 |
| WNT3A|1 | P56704|1 | 200 | 0.1 |
| Wnt5a|1 | P22725|1 | 100 | 0.1 |
Table 1: The full list of ligands used for the MEMA experiments. The uniprot ID, stock concentrations, and final working concentrations are provided.
| ECM Protein | UniprotID | Stock Concentration (µg/mL) |
Final Concentration (µg/mL) |
Notes |
| ALCAM|1 | Q13740|1 | 100 | 30 | |
| CDH20 | Q9HBT6 | 300 | 80 | |
| CDH6|1 | P55285|1 | 100 | 40 | |
| CDH8 | P55286 | 100 | 20 | |
| CD44|1 | P16070|1 | 100 | 30 | |
| CEACAM6 | P40199 | 100 | 30 | |
| COL1A1 | P02453 | 5000 | 200 | multiple subunits with multiple uniprot ids |
| COL2A1|2 | P02458|2 | 1000 | 200 | |
| COL3A1|1 | P02461|1 | 1000 | 200 | |
| COL4A1|1 | P02462|1 | 1000 | 200 | multiple subunits with multiple uniprot ids |
| COL5A1 | P20908 | 1000 | 200 | |
| COL23A1|1 | Q86Y22|1 | 200 | 80 | |
| DSG2 | Q14126 | 100 | 30 | |
| CDH1|1 | P12830|1 | 100 | 40 | |
| ECM1|1 | Q16610|1 | 100 | 40 | |
| FN1|1 | P02751|1 | 1000 | 200 | |
| GAP43|1 | P17677|1 | 158 | 40 | |
| HyA-500K | 1000 | 200 | LOR-0005 | |
| HyA-50K | 1000 | 200 | LOR-0007 | |
| ICAM1 | P05362 | 400 | 80 | |
| ALCAM|1 | Q13740|1 | 100 | 30 | |
| CDH20 | Q9HBT6 | 300 | 80 | |
| CDH8 | P55286 | 100 | 20 | |
| CD44|1 | P16070|1 | 100 | 30 | |
| CEACAM6 | P40199 | 100 | 30 | |
| DSG2 | Q14126 | 100 | 30 | |
| CDH15 | P55291 | 100 | 20 | |
| VCAM1|1 | P19320|1 | 1000 | 200 | |
| LAMA1 | P25391 | 500 | 200 | multiple subunits with multiple uniprot ids |
| LAMA3|2 | Q16787|2 | 130 | 40 | |
| LUM | P51884 | 200 | 80 | |
| CDH15 | P55291 | 100 | 20 | |
| NID1|1 | P14543|1 | 100 | 9.3 µg/mL Nid, 130 µg/mL Lam, 46.5 µg/mL COL4 | +COL4 and laminin |
| OMD | Q99983 | 100 | 40 | |
| SPP1|A | P10451|1 | 100 | 40 | |
| CDH3|1 | P22223|1 | 100 | 40 | |
| PECAM1|Long | P16284|1 | 150 | 40 | |
| TNC|1 | P24821|1 | 500 | 200 | |
| VCAM1|1 | P19320|1 | 1000 | 200 | |
| VTN | P04004 | 100 | 40 | |
| BGN | P21810 | 100 | 40 | |
| DCN|A | P07585|1 | 300 | 80 | |
| POSTN|1 | Q15063|1 | 100 | 40 | |
| SPARC | P09486 | 100 | 40 | |
| THBS1|1 | P07996|1 | 100 | 40 | |
| BCAN|1 | Q96GW7|1 | 100 | 40 | |
| ELN|3 | P15502|3 | 1000 | 200 | |
| FBN1 | P35555 | 254 | 80 |
Table 2: The full list of ECM proteins and conditions that are used in the MEMA experiments. The uniprot ID, stock concentrations, and final working concentrations are provided. In some instances, the printed condition represents a protein complex or a combination of multiple proteins, which is indicated in the Notes column.
Discussion
The importance of "dimensionality" and context has been a motivating factor in the development of in vitro culture systems as tools in the characterization of cancer cells through their interaction with the microenvironment11, and the ability of in vitro culture systems to mimic the in vivo environment is a driving force behind the quest to improve those culture systems. In vitro systems, however, remain significant tools of cancer research precisely because of their ability to distill the complex in vivo situation down to a simplified model12.
Although 2D systems can include ECMs and ligands, they have traditionally lacked the throughput capacities to interrogate a wide panel of combinatorial pertubagens. Popular commercial basement membrane extracts allow for culturing in 3D, but lack the provenance of a carefully defined panel of proteins. The commercial extracts typically suffer from incompletely defined composition, which can confound analysis and result in significant batch-to-batch variation3,13. The MEMA platform overcomes these barriers, allowing for the study of alterations in cellular phenotypes, metabolic activity, differentiation status, and variations in cell growth and proliferation as they are modulated by specific and defined endogenous factors.
The MEMA platform is a powerful, medium- to high-throughput approach to assess the impact of the microenvironment (both ECM and soluble factors) on the phenotype of cells. The platform shows great flexibility for the types of assays and cells for which it can be utilized. We can observe effects from both soluble ligands and the ECM proteins to which the cells are exposed. Indeed, we recently found that ligands were a major driver of resistance to HER2-targeted inhibitors, but that these effects could be modulated by the ECM5. A variety of cells, including primary cells and cell lines derived from different cell types including lung, bladder, prostate, breast, and pancreas, as well as induced pluripotent stem (iPS) cells, have been successfully cultured on the MEMA platform (see examples in references5,7,14). The use of different stains allows for the readout of multiple cellular endpoints, including cell growth, differentiation, and metabolism. Other researchers have extended the platform to interrogate the impact of stiffness or elastic modulus, adding an additional dimension to the MEMA platform15. Finally, the platform is amenable to performing drug screens for identification of microenvironment conditions that either enhance or inhibit drug efficacy, as we and others have recently reported5,14,15.
Perhaps the most critical step to the success of a MEMA experiment is optimizing the cell plating density. Optimizing the density of the cells ensures that enough cells are present to provide robust data, but not so many that the spot becomes overly confluent. Confluent spots can significantly confound results, particularly if proliferation is used as an endpoint, making it impossible to determine if low proliferation rates are a result of interactions with microenvironmental factors or due to contact inhibition from high cellular density. Cell titration experiments can reveal these problems, as average cell numbers per spot will demonstrate a linear increase with increasing numbers of cells plated, but will eventually plateau. The optimal cell number should be chosen in the linear range of the curve.
As mentioned above, the MEMA platform is flexible and can be prepared on a variety of substrates with different surfaces. These include glass slides and multiwall plate formats. In our experience, not all surface chemistries are amenable to MEMA printing, as we have observed spot detachment on some surfaces due to poor adhesion properties and the inability to block cell adhesion on other high adhesion surfaces. Furthermore, changing between different substrates necessitates optimization of buffer conditions, as the performance of the printing with the same print buffer can vary depending on surface chemistry.
The diameter of the printed ECM spots plays an important role in the quality of the data. In general, we recommend using the largest diameter print pins available for the arrayer in use (we currently use 350 µm diameter pins). Larger diameter spots allow a greater number of cells to occupy a spot, which tends to result in more robust data than are generated with smaller diameter pins. Since binding of the cells is a stochastic process, there does tend to be a high degree of variability in the data that is related to the number of cells originally attached to each spot. Thus, we recommend printing a large number of replicates for each ECM condition. We print 10−15 ECM replicates in each well with our current print conditions to ensure robust statistics.
We have noted in our past experiments that for the most part, ligand effects tend to dominate over ECM effects. This may be in part due to our decision to add collagen I to all ECM spots, which ensures robust cell binding. However, we believe that this may also homogenize the ECM effects, as most spots tend to behave in a manner highly similar to collagen I. Altering the spot composition to exclude collagen I may result in differential cell behavior as a result of the interaction with the ECM, but also significantly impacts cell binding, resulting in many more unoccupied spots. Users should tailor their ECM composition keeping these differences in mind, particularly those interested in stem and progenitor cells and differentiation, where the matrix can have a significant impact16.
We typically perform the MEMA assays for relatively short periods of time (e.g., 72 h maximum). This is because the cells are constrained to the spots (the blocking buffer does not allow for growth outside the spots in our experience). With rapidly dividing cells, growth longer than 72 h will lead to overgrowth of the spot, which in turn complicates image segmentation as cells become crowded and pile up on each other, and can also impact data as growth arrest can occur with contact inhibition. We have performed longer treatments with very slow growing primary cells (10−14 days), but care must be taken in these assays to change the media and replenish ligands every 3−4 days.
Continuing efforts to develop the MEMA platform are focused on two areas of interest, maximization of the optical quality for imaging and optimization within smaller culture vessels. Optical quality becomes a crucial factor when researchers require higher resolution microscopy to identify subcellular localization of their markers of interest. Initial screens can be performed at lower resolution on high-throughput microscopes followed by imaging of specific spots of interest on higher resolution instruments, but image quality can be compromised if the optical properties of the substrate are poor. Improvement of the optical properties of the substrate would allow researchers to perform the initial screens on high resolution imaging systems without the need to reacquire selected images at higher resolution. Finally, the ability to perform MEMAs in smaller culture vessels, such as 96-well plates, would allow a reduction in treatment volume and an expansion of interrogated ligands and replicates. This transition requires the optimization of substrate-buffer-protein interactions and array printing within new culture vessels. Such ongoing efforts will improve the MEMA platform and expand upon its powerful capabilities to identify relevant microenvironmental proteins that alter cellular phenotypes for a variety of cell types, which can then be subsequently investigated in confirmatory assays.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The work in this manuscript was supported by the NIH Common Fund Library of Network Cellular Signatures (LINCS) U54 grant HG008100 (J.W.G., L.M.H., and J.E.K) and NCI Cancer Systems Biology Consortium (CSBC) U54 CA209988grant (J.W.G., L.M.H., and J.E.K).
Materials
| Aushon 2470 | Aushon BioSystems | Arrayer robot system used in the protocol | |
| Nikon HCA | Nikon | High Content Imaging system designed around Nikon Eclipse Ti Inverted Microscope | |
| BioTek Precision XS liquid Handler | BioTek | liquid handling robot used in the protocol | |
| Trizma hydrochloride buffer solution | Sigma | T2069 | |
| EDTA | Invitrogen | 15575-038 | |
| Glycerol | Sigma | G5516 | |
| Triton X100 | Sigma | T9284 | |
| Tween 20 | Sigma | P7949 | |
| Kolliphor P338 | BASF | 50424591 | |
| 384-well microarray plate, cylindrical well | Thermo Fisher | ab1055 | |
| Nunc 8 well dish | Thermo Fisher | 267062 | |
| Paraformaldehyde 16% solution | Electron Microscopy Science | 15710 | |
| BSA | Fisher | BP-1600 | |
| Sodium Azide | Sigma | S2002 | |
| Cell Mask | Molecular Probes | H32713 | |
| Click-iTEdU Alexa Fluor | Molecular Probes | C10357 | |
| DAPI | Promo Kine | PK-CA70740043 | |
| ALCAM | R & D Systems | 656-AL | ECM |
| Cadherin-20 (CDH20) | R & D Systems | 5604-CA | ECM |
| Cadherin-6 (CDH6) | R & D Systems | 2715-CA | ECM |
| Cadherin-8 (CDH8) | R & D Systems | 188-C8 | ECM |
| CD44 | R & D Systems | 3660-CD | ECM |
| CEACAM6 | R & D Systems | 3934-CM | ECM |
| Collagen I | Cultrex | 3442-050-01 | ECM |
| Collagen Type II | Millipore | CC052 | ECM |
| Collagen Type III | Millipore | CC054 | ECM |
| Collagen Type IV | Sigma | C5533 | ECM |
| Collagen Type V | Millipore | CC077 | ECM |
| COL23A1 | R & D Systems | 4165-CL | ECM |
| Desmoglein 2 | R & D Systems | 947-DM | ECM |
| E-cadherin (CDH1) | R & D Systems | 648-EC | ECM |
| ECM1 | R & D Systems | 3937-EC | ECM |
| Fibronectin | R & D Systems | 1918-FN | ECM |
| GAP43 | Abcam | ab114188 | ECM |
| HyA-500K | R & D Systems | GLR002 | ECM |
| HyA-50K | R & D Systems | GLR001 | ECM |
| ICAM-1 | R & D Systems | 720-IC | ECM |
| Laminin | Sigma | L6274 | ECM |
| Laminin-5 | Abcam | ab42326 | ECM |
| Lumican | R & D Systems | 2846-LU | ECM |
| M-Cad (CDH15) | R & D Systems | 4096-MC | ECM |
| Nidogen-1 | R & D Systems | 2570-ND | ECM |
| Osteoadherin/OSAD | R & D Systems | 2884-AD | ECM |
| Osteopontin (SPP) | R & D Systems | 1433-OP | ECM |
| P-Cadherin (CDH3) | R & D Systems | 861-PC | ECM |
| PECAM1 | R & D Systems | ADP6 | ECM |
| Tenascin C | R & D Systems | 3358-TC | ECM |
| VCAM1 | R & D Systems | ADP5 | ECM |
| vitronectin | R & D Systems | 2308-VN | ECM |
| Biglycan | R & D Systems | 2667-CM | ECM |
| Decorin | R & D Systems | 143-DE | ECM |
| Periostin | R & D Systems | 3548-F2 | ECM |
| SPARC/osteonectin | R & D Systems | 941-SP | ECM |
| Thrombospondin-1/2 | R & D Systems | 3074-TH | ECM |
| Brevican | R & D Systems | 4009-BC | ECM |
| Elastin | BioMatrix | 5052 | ECM |
| Fibrillin | Lynn Sakai Lab OHSU | N/A | ECM |
| ANGPT2 | RnD_Systems_Own | 623-AN-025 | Ligand |
| IL1B | RnD_Systems_Own | 201-LB-005 | Ligand |
| CXCL8 | RnD_Systems_Own | 208-IL-010 | Ligand |
| IGF1 | RnD_Systems_Own | 291-G1-200 | Ligand |
| TNFRSF11B | RnD_Systems_Own | 185-OS | Ligand |
| BMP6 | RnD_Systems_Own | 507-BP-020 | Ligand |
| FLT3LG | RnD_Systems_Own | 308-FK-005 | Ligand |
| CXCL1 | RnD_Systems_Own | 275-GR-010 | Ligand |
| DLL4 | RnD_Systems_Own | 1506-D4-050 | Ligand |
| HGF | RnD_Systems_Own | 294-HGN-005 | Ligand |
| Wnt5a | RnD_Systems_Own | 645-WN-010 | Ligand |
| CTGF | Life_Technologies_Own | PHG0286 | Ligand |
| LEP | RnD_Systems_Own | 398-LP-01M | Ligand |
| FGF2 | Sigma_Aldrich_Own | SRP4037-50UG | Ligand |
| FGF6 | RnD_Systems_Own | 238-F6 | Ligand |
| IL7 | RnD_Systems_Own | 207-IL-005 | Ligand |
| TGFB1 | RnD_Systems_Own | 246-LP-025 | Ligand |
| PDGFB | RnD_Systems_Own | 220-BB-010 | Ligand |
| WNT10A | Genemed_Own | 90009 | Ligand |
| PTN | RnD_Systems_Own | 252-PL-050 | Ligand |
| BMP3 | RnD_Systems_Own | 113-BP-100 | Ligand |
| BMP4 | RnD_Systems_Own | 314-BP-010 | Ligand |
| TNFSF11 | RnD_Systems_Own | 390-TN-010 | Ligand |
| CSF2 | RnD_Systems_Own | 215-GM-010 | Ligand |
| BMP5 | RnD_Systems_Own | 615-BMC-020 | Ligand |
| DLL1 | RnD_Systems_Own | 1818-DL-050 | Ligand |
| NRG1 | RnD_Systems_Own | 296-HR-050 | Ligand |
| KNG1 | RnD_Systems_Own | 1569-PI-010 | Ligand |
| GPNMB | RnD_Systems_Own | 2550-AC-050 | Ligand |
| CXCL12 | RnD_Systems_Own | 350-NS-010 | Ligand |
| IL15 | RnD_Systems_Own | 247-ILB-005 | Ligand |
| TNF | RnD_Systems_Own | 210-TA-020 | Ligand |
| IGFBP3 | RnD_Systems_Own | 675-B3-025 | Ligand |
| WNT3A | RnD_Systems_Own | 5036-WNP-010 | Ligand |
| PDGFAB | RnD_Systems_Own | 222-AB | Ligand |
| AREG | RnD_Systems_Own | 262-AR-100 | Ligand |
| JAG1 | RnD_Systems_Own | 1277-JG-050 | Ligand |
| BMP7 | RnD_Systems_Own | 354-BP-010 | Ligand |
| TGFB2 | RnD_Systems_Own | 302-B2-010 | Ligand |
| VEGFA | RnD_Systems_Own | 293-VE-010 | Ligand |
| IL6 | RnD_Systems_Own | 206-IL-010 | Ligand |
| CXCL12 | RnD_Systems_Own | 351-FS-010 | Ligand |
| NRG1 | RnD_Systems_Own | 378-SM | Ligand |
| IGFBP2 | RnD_Systems_Own | 674-B2-025 | Ligand |
| SHH | RnD_Systems_Own | 1314-SH-025 | Ligand |
| FASLG | RnD_Systems_Own | 126-FL-010 | Ligand |
References
- Hanahan, D., Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 21 (3), 309-322 (2012).
- Quail, D. F., Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nature Medicine. 19 (11), 1423-1437 (2013).
- Hughes, C. S., Postovit, L. M., Lajoie, G. A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 10 (9), 1886-1890 (2010).
- LaBarge, M. A., et al. Human mammary progenitor cell fate decisions are products of interactions with combinatorial microenvironments. Integrative Biology (Cambridge). 1 (1), 70-79 (2009).
- Watson, S. S., et al. Microenvironment-Mediated Mechanisms of Resistance to HER2 Inhibitors Differ between HER2+ Breast Cancer Subtypes. Cell Systems. 6 (3), 329-342 (2018).
- Ranga, A., et al. 3D niche microarrays for systems-level analyses of cell fate. Nature Communications. 5, 4324 (2014).
- Malta, D. F. B., et al. Extracellular matrix microarrays to study inductive signaling for endoderm specification. Acta Biomater. 34, 30-40 (2016).
- Kamentsky, L., et al. Improved structure, function and compatibility for CellProfiler: modular high-throughput image analysis software. Bioinformatics. 27 (8), 1179-1180 (2011).
- Gagnon-Bartsch, J. A., Jacob, L., Speed, T. P. Removing Unwanted Variation from High Dimensional Data with Negative Controls. University of California, Berkeley, Department of Statistics, University of California, Berkeley. , (2013).
- Allan, C., et al. OMERO: flexible, model-driven data management for experimental biology. Nature Methods. 9 (3), 245-253 (2012).
- Simian, M., Bissell, M. J. Organoids: A historical perspective of thinking in three dimensions. Journal of Cell Biology. 216 (1), 31-40 (2017).
- Bissell, M. J. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. International Review of Cytology. 70, 27-100 (1981).
- Serban, M. A., Prestwich, G. D. Modular extracellular matrices: solutions for the puzzle. Methods. 45 (1), 93-98 (2008).
- Kaylan, K. B., et al. Mapping lung tumor cell drug responses as a function of matrix context and genotype using cell microarrays. Integrative Biology (Cambridge). 8 (12), 1221-1231 (2016).
- Lin, C. H., Jokela, T., Gray, J., LaBarge, M. A. Combinatorial Microenvironments Impose a Continuum of Cellular Responses to a Single Pathway-Targeted Anti-cancer Compound. Cell Reports. 21 (2), 533-545 (2017).
- Gjorevski, N., et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 539 (7630), 560-564 (2016).

