Monitoring Bacterial Colonization and Maintenance on Arabidopsis thaliana Roots in a Floating Hydroponic System
Summary
Here we describe a hydroponic plant growth assay to quantify species presence and visualize the spatial distribution of bacteria during initial colonization of plant roots and after their transfer into different growth environments.
Abstract
Bacteria form complex rhizosphere microbiomes shaped by interacting microbes, larger organisms, and the abiotic environment. Under laboratory conditions, rhizosphere colonization by plant growth-promoting bacteria (PGPB) can increase the health or the development of host plants relative to uncolonized plants. However, in field settings, bacterial treatments with PGPB often do not provide substantial benefits to crops. One explanation is that this may be due to loss of the PGPB during interactions with endogenous soil microbes over the lifespan of the plant. This possibility has been difficult to confirm, since most studies focus on the initial colonization rather than maintenance of PGPB within rhizosphere communities. It is hypothesized here that the assembly, coexistence, and maintenance of bacterial communities are shaped by deterministic features of the rhizosphere microenvironment, and that these interactions may impact PGPB survival in native settings. To study these behaviors, a hydroponic plant-growth assay is optimized using Arabidopsis thaliana to quantify and visualize the spatial distribution of bacteria during initial colonization of plant roots and after transfer to different growth environments. This system’s reproducibility and utility are then validated with the well-studied PGPB Pseudomonas simiae. To investigate how the presence of multiple bacterial species may affect colonization and maintenance dynamics on the plant root, a model community from three bacterial strains (an Arthrobacter, Curtobacterium, and Microbacterium species) originally isolated from the A. thaliana rhizosphere is constructed. It is shown that the presence of these diverse bacterial species can be measured using this hydroponic plant-maintanence assay, which provides an alternative to sequencing-based bacterial community studies. Future studies using this system may improve the understanding of bacterial behavior in multispecies plant microbiomes over time and in changing environmental conditions.
Introduction
Crop destruction by bacterial and fungal diseases results in lowered food production and can severely disrupt global stability1. Based on the discovery that microbes in suppressive soils are responsible for increasing plant health2, scientists have asked whether the plant microbiome can be leveraged to support plant growth by modifying the presence and abundance of particular bacterial species3. Bacteria found to aid in plant growth or development are collectively termed plant growth-promoting bacteria (PGPB). More recently, studies have shifted from simply identifying potential PGPB to understanding how interkingdom interactions in the soil, around roots, or in the rhizosphere (the area directly surrounding and including the root surface) may be impacting PGPB activity4.
Rhizosphere colonization by PGPB can increase the health or the development of host plants in response to diverse stressors relative to uncolonized plants5. However, results are often more variable in native soil conditions compared to those observed in the closely controlled greenhouse and laboratory settings6. One hypothesis for this difference is that the growth or behavior of PGPB may be inhibited by native soil bacteria or fungi in the fields7,8. Beneficial effects by rhizosphere bacteria generally depend on the ability of the bacteria to 1) locate and move towards the root, 2) colonize the root through biofilm formation, and 3) interact with the host plant or pathogens via production of small molecule metabolites7,9. Any of these colonization behaviors may be affected by the presence and activity of neighboring microbes10.
We designed a system to quantify and visualize these distinct bacterial colonization stages of the rhizosphere (Figure 1). This approach will facilitate studies investigating why long-term PGPB maintenance is sometimes not observed following transfer of plants into new environments, such as during the planting of pre-inoculated seedlings. Arabidopsis thaliana as were chosen as a plant model due to its extensive use in laboratory studies as well as the ample data available about its microbial interactions11. There are three stages in the system: 1) A. thaliana growth, 2) bacterial colonization, and 3) bacterial maintenance (see Figure 1). Because A. thaliana is a terrestrial plant, it was important to ensure that it was not suffering undue water stress in the hydroponic system12. Inspired by the methods used by Haney et al.13, the seedlings are grown on plastic mesh to separate the shoot from the liquid growth medium. This system does not appear to compromise the health and development of the plant host, and it improves A. thaliana growth in liquid11. As the plant shoot floats above the surface, the roots are fully exposed to colonization by bacteria inoculated into the liquid bacterial growth medium. This permits bacteria of interest to be examined for colonization in nutrients that are most conducive to growth, while then shifting conditions to allow the plant to continue growing in a nutrient medium designed to support its growth. Both stages include steady shaking to prevent anoxia of the root13. Bacteria can be visualized or quantified from the plant roots following transfer from either the colonization medium or the maintenance medium. This hydroponic system is very flexible, allowing experimental conditions and applied stresses to be easily altered depending on interests of the researchers.
This described method is important in the context of the larger body of literature about plant-microbe interactions because it provides a robust system for studying these interactions at the root surface while also being customizable to the growth preferences of different bacteria. Plant biology labs often perform plant-microbe colonization experiments on solid agar, allowing for only planar movement (if that) of bacteria while also requiring the potentially destructive manipulation of plants during subsequent transfer. In contrast, microbiology labs have frequently prioritized the health of the bacteria within their experiments, to the detriment of the plants14,15. These different priorities of plant- and microbiology-focused labs have historically made it difficult to compare results between these groups, since each typically optimizes experimental conditions to optimize their organism of interest15. The floating-mesh-plant-growth system described here prevents full plant submersion, a notable advantage to previous microbiology-oriented studies, while also temporarily optimizing the growth and survival of bacteria to facilitate colonization. Thus, the assay we present here may address concerns from both plant biologists (about over-hydration and tactile manipulation of the plant) while satisfying the criteria of microbiologists (allowing for different bacterial growth conditions and multiple species’ interactions)7. This protocol is designed to be adaptable for use with various bacteria, plants, and environmental conditions.
Protocol
NOTE: The experimental setup is described for clarity and used to generate the representative results included in this report, but conditions can be modified as desired. All steps should be performed using PPE and following institutional and federal reccomendations for safety, according to the BSL status of the bacteria used.
1. Characterization of bacteria
- Determine the morphology of bacteria on the growth medium agar plate. Resuspend cells at an approximate OD600 = 0.5 and plate a 1 µL volume onto agar medium of choice. Add X-gal to agar plates to a final concentration of 20 mg/mL to better differentiate individual members of the specific bacterial community. Grow at 24 °C or 30 °C until colonies form, then take pictures of and notes on colony morphology.
- Define the correlation between each bacterial strain’s optical density and the number of CFU (colony forming units) per mL16. Resuspend bacteria in 1 mL of water in a 24-well plate to an approximate OD600 = 5, perform two-fold serial dilutions, monitor OD600 of all dilutions, and plate each to determine the viable CFU/mL in each sample at multiple optical densities.
- Determine the maximum sonication tolerance for each bacterial strain. To do this, aliquot cells into a 24-well plate containing liquid medium, reserving some cells as an unsonicated control sample. Using an ultrasonicator with a 24-tip horn attachment, apply three rounds of 12 s of sonication at 40 amp with 2 s pulses.
NOTE: The use of a 24-well ultrasonicator is advised to facilitate the downstream multiplexing of processing plant samples, but if one is not available, use an ultrasonicator fitted with a microtip and perform each sample sonication independently. Always wear earmuffs rated to at least 25 NRR protection. - Perform 10-fold serial dilutions of the sonicated and unsonicated samples and spot onto agar plates. Determine whether there is a reduction in viable cells after sonication. If so, use a fresh sample and repeat sonication step using a reduced total sonication time or amplitude until the treatment has no effect on final CFU/mL17.
2. Preparation of Arabidopsis thaliana seedlings on a plastic mesh
- Create disks of the plastic mesh using a standard hole puncher.
- Collect the disks in a glass container with a loose cover of aluminum foil, and sterilize using an autoclave set to a 20 min dry cycle13.
- Using flame-sterilized tweezers, distribute approximately 40 sterilized mesh disks in a single layer across the surface of a plant-growth-medium agar plate. Use 0.5x Murashige and Skoog (MS) salts, containing 500 mg/L of MES buffer [2-(N-morpholino)ethanesulfonic acid] and 1.5% Bacto agar, as plant growth medium, with 50 µg/mL benomyl added to the limit fungal contamination of the seedlings.
- Prepare axenic seeds of A. thaliana as previously described17.
- Place approximately 100–300 seeds each into individual centrifuge tubes in a rack and place into a resealable glass or heavy plastic container (“jar”) in a fume hood.
- Using caution, place a beaker of 100 mL of bleach into the jar, add 3 mL of concentrated HCl to the bleach, and immediately seal the jar and allow fumes to sterilize seeds for at least 4 h.
- Carefully remove the tubes of sterilized seeds from underneath the jar and seal.
- Place two seeds at the center of each mesh. Seal plates with surgical tape and incubate for 2–6 days at 4 °C in darkness to vernalize seeds.
- To germinate and grow seedlings, place the plate agar side down in a plant growth chamber for 8–10 days under short day settings: 9 h of light at 21 °C and 15 h of dark at 18 °C (Figure 1, step 2).
3. Colonization of plants in liquid bacterial growth medium
- Add 1 mL of bacterial growth medium to each well of a sterile 24-well plate, except for media-only control wells. Use Lennox Luria Broth (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl) as the bacterial growth medium.
- Transfer the germinated seedlings embedded in mesh from agar plates to the liquid (Figure 1, step 3a).
- Gently peel the mesh containing two germinated seedlings up and off the agar plate using flame-sterilized forceps. Choose mesh with equally sized and undamaged seedlings.
- If removal from the agar is not smooth, discard that mesh and plant. Transfer one float to each well of bacterial growth liquid, root side down.
- Inoculate bacteria into wells containing floating seedlings.
- Resuspend bacteria grown overnight on agar plates to an OD600 equivalent to 108 CFU/mL in the bacterial growth medium liquid. Add 10 µL of bacterial suspension to each well for a final concentration of 106 CFU bacteria per well.
- If preparing a mix of bacteria, resuspend each to the OD600 equivalent to 108 CFU/mL, mix in equal proportions, and add 10 µL of the final mix per well of liquid.
- Seal the plate for sterile growth. Without touching the sticky side, carefully press the gas-permeable film across the plate. Ensure that each well has been individually sealed by applying pressure around each of the rings made by the wells. Replace the plate’s plastic lid snuggly over the plate and gas-permeable film (Figure 1, step 3b).
- Incubate the plates for 18 h in a plant growth chamber, under the same conditions as the seedlings were originally germinated, except on an orbital plate shaker set to 220 rpm.
4. Maintenance of bacterial colonization
- To rinse all floats (plants on mesh), add 1 mL of sterile water to wells of a new 24-well plate. Remove gas-permeable film. Using sterile forceps, transfer floats to wells with water (Figure 1, step 4a). Rinse by resting for 10 min at room temperature (RT) without agitation.
NOTE: To determine bacterial colonization efficiency of roots rather than their ability to maintain colonization over time, plants can be sacrificed at this step by taking them directly to step 5.1. - Fill the wells of a new 24-well plate with 1 mL of plant growth medium. Transfer one mesh to each well. Cover with a gas-permeable seal and incubate for 72 h on the orbital plate shaker at 220 rpm in plant growth chamber (Figure 1, step 4b).
- Repeat the rinsing as performed in step 4.1 with floats after the 72 h incubation period.
5. Collection of bacteria for viable cell counts
NOTE: The number of bacteria per seedling root can be determined at any incubation timepoint. Colonization can be monitored between 0 h and 18 h, while maintenance can be monitored from 18 h onwards. Plants destined for imaging can proceed directly to section 6.
- Remove the seedlings from the mesh (Figure 1, step 5). Gently place flame-sterilized forceps below the leaves (but on the leaf side of the mesh), and lightly pinch the stem. Wiggle the seedlings up and away from the mesh to dislodge the root without breaking it. If the root breaks, gently scrape the mesh bottom to collect the full length.
- Remove bacteria from plant roots. Transfer the bacteria to wells of a 24-well plate containing 1 mL of ddH20. Sonicate the samples as described in step 1.3.
NOTE: Using a microscope, look for any remaining bacteria on the root surface on a sonicated sample. If bacteria remain, increase the total sonication time or intensity until no bacteria remain bound, up to the highest level of sonication that does not affect viable cell counts as determined in section 1. - Quantify the bacteria on roots.
- Perform serial 10-fold dilutions of the sonicated samples up to a 10-6 dilution in bacterial growth medium. Add 50 µL of each dilution to individual agar plates and spread with sterile glass beads (or bacterial spreader). Incubate plates at the optimal temperature for bacteria until individual colonies are countable.
- Once distinguishable, count the number of each colony morphology (as determined in section 1), and calculate CFU of each bacterial species per seedling. Discard any samples showing contamination, as contamination during colonization or maintenance may affect bacterial presence.
6. Collection of intact plant roots for microscopy
- Using forceps, remove the seedlings from mesh as in section 5.
- Transfer each plant to microscope slides.
- Place the tip of the root on the slide and drag away from the tip to set the shoot down flush with the slide, ensuring a straightened root for best imaging. Add a drop of water or sterile plant growth medium to the samples to hydrate interfaces between the coverslips and slides.
- Place a glass coverslip just above the root crown (uppermost boxed region in Figure 1) and below the shoot leaves to avoid slanting of the coverslip (to allow for root crown imaging), and press down gently17.
- If using fluorescent bacteria, image using appropriate excitation/emission filters to differentiate bacteria from each other and the plant root18.
Representative Results
The well-characterized PGPB P. simiae WCS417r is known to colonize the roots of A. thaliana in hydroponic culture. This naturally fluorescent bacterium can easily be visualized using microscopy on the roots of seedlings following colonization (Figure 2). Although it is possible to image the full length of these A. thaliana seedlings’ (4–6 mm length) roots, doing so for many plants would take a prohibitive amount of time. Because most variation across timepoints and species of bacteria can be captured by imaging the crown, middle, and tip of the root14 (indicated by red boxes in Figure 1), these regions were prioritized for imaging rather than imaging full root lengths. In the bright-field images of P. simiae-colonized A. thaliana roots (Figure 2), it is possible to visualize the outline of the roots and root hairs; however, at 18 h of colonization, it is not possible to clearly differentiate colonized versus non-colonized roots using bright-field images. While P. simiae displays autofluorescence, we used a strain also engineered to express a yellow fluorescent protein (YFP)19 with excitation/emission wavelengths of 490-510/520-550 nm18. A magnification of 100x was sufficient to clearly identify individual and small aggregates of P. simiae cells on A. thaliana roots. As shown in Figure 2, laboratories with access to either high-resolution confocal microscopes or less expensive benchtop microscopes can both visualize the presence and distribution of bacteria along the root.
While informative in terms of spatial distribution, microscopy images are not well-suited for quantification of bacterial cells. We thus collected bacteria from the surface of roots using ultrasonication as previously described and validated9,20. Three rounds of 12 s of ultrasonication21 at an amplitude of 40 were sufficient to disrupt the outer surface of the root seedlings (Supplemental Figure 1) and remove all bacteria while not impacting the bacterial viability. Sonication was used rather than bead-beating methods9 to better promote dispersal of bacterial aggregates/biofilms. Quantifying CFU/root after 18 h of colonization and an additional 72 h of maintenance showed that P. simiae both colonizes and is maintained on the roots of A. thaliana in our hydroponic, floating seedling system (Figure 3). The number of CFU/seedling at either timepoint showed good reproducibility across biological replicates performed on different days (Figure 3). The variation observed is common among root colonization assays22 and is likely due to minor variations of timing, environmental conditions, or plant root size, even among seedlings germinated at the same time and selected to be similar in size. We observe an increase in the number of CFU/seedling after 72 h in maintenance medium as compared to the numbers observed at the post-colonization 18 h timepoint (Figure 3). This indicates active growth of the colonized bacteria on the plant root occurred during the maintenance stage.
In addition to the utility of this hydroponic assay to quantify individual bacterial colonization and maintenance, it is also applicable to monitoring the association of multiple species on plant roots. To demonstrate this, three species of bacteria isolated from A. thaliana grown in natural soil under laboratory conditions were chosen20. The isolates were strains of Arthrobacter nicotinovorans, Microbacterium oleivorans, and Curtobacterium oceanosedimentum23. This simplified community was chosen due to these species’ ability to coexist in liquid bacterial growth media in shaking culture (unpublished data). In addition, these three species can be clearly differentiated on media containing X-gal due to differences in colony morphology and color (Figure 4A). The X-gal does not affect relative growth of any of these bacterial species (unpublished data). These differences in morphology and colony appearance on X-gal allowed us to count the CFU/seedling of each species without antibiotic selection, even in multi-species coculture.
A. nicotinovorans, M. oleivorans, and C. oceanosedimentum were all colonized and maintained on the root, whether alone or in bacterial coculture (Figure 4B). Each species showed trends that were similar across different biological and technical replicates, even within mixed communities. This demonstrates that the assay protocol can be used to measure both relative or total CFU/root of each species. Interestingly, when grown alone, no individual species showed an appreciable increase in abundance during the maintenance stage, but the overall CFU/root of the combined community increased in cocultures, indicating that these bacteria do not prohibit the colonization of the other strains.
For all experiments, plants grown in liquid media without the addition of bacteria as negative controls were always included. No bacteria were visible on these control roots during microscopy (Figure 2), nor were any bacteria detected via plating for CFU (unpublished data). This indicates that sterilization of seeds and using the sterile techniques during this assay were sufficient to keep plants axenic unless purposely colonized.
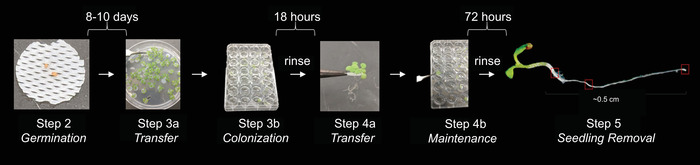
Figure 1: Assay for bacterial colonization and maintenance on A. thaliana roots. A. thaliana seedlings grown on sterilized plastic mesh were transferred to a growth medium optimized for bacteria [here, 0.1 x LB (Luria Broth) Lennox]. Bacteria then colonized the root over 18 h while the plant floated in shaking liquid. Following a rinse, the colonized float was transferred to a growth medium optimized for plants (0.5x MS + MES) for 72 h to test for maintenance of bacteria on the roots. The float was then rinsed, and the plant with any attached microbes was removed for analysis (quantification of CFU/seedling or imaging by microscopy). Please click here to view a larger version of this figure.

Figure 2: Visualizing P. simiae colonization of roots with fluorescent microscopy. P. simiae (false-colored green) colonized A. thaliana roots and was maintained on the root following transfer to plant-growth medium. Root crown (left), mid-length (center), and tip (right) at 40x magnification are shown from areas indicated in Figure 1. The top two rows show the bright-field and fluorescent images of roots colonized by P. simiae (imaged by epifluorescent microscopy). The same roots were also imaged by a confocal microscope (center two rows). The no-bacteria negative control in the two bottom rows showed no colonization. Scale bars represent 50 µm. Please click here to view a larger version of this figure.
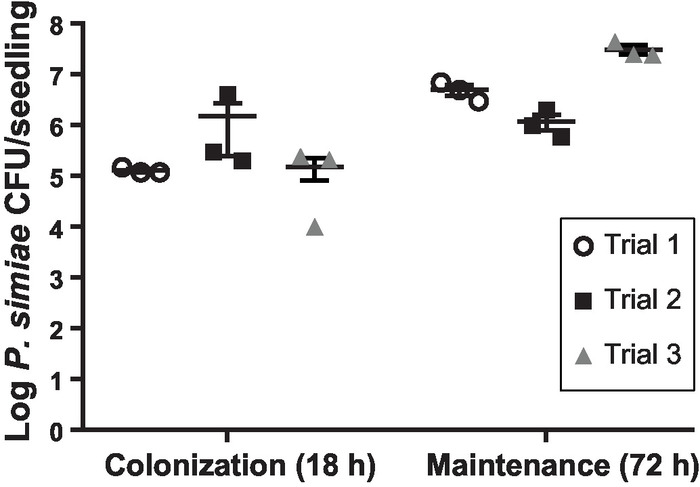
Figure 3: Quantification of P. simiae on A. thaliana roots. Total number of P. simiae viable cells recovered per A. thaliana seedling following 18 h of colonization or 72 h of maintenance. Three individual biological replicates are shown, each containing three technical replicates of two seedlings per float. The numbers shown are the means from the technical replicates, while bars represent standard errors of the means. Please click here to view a larger version of this figure.
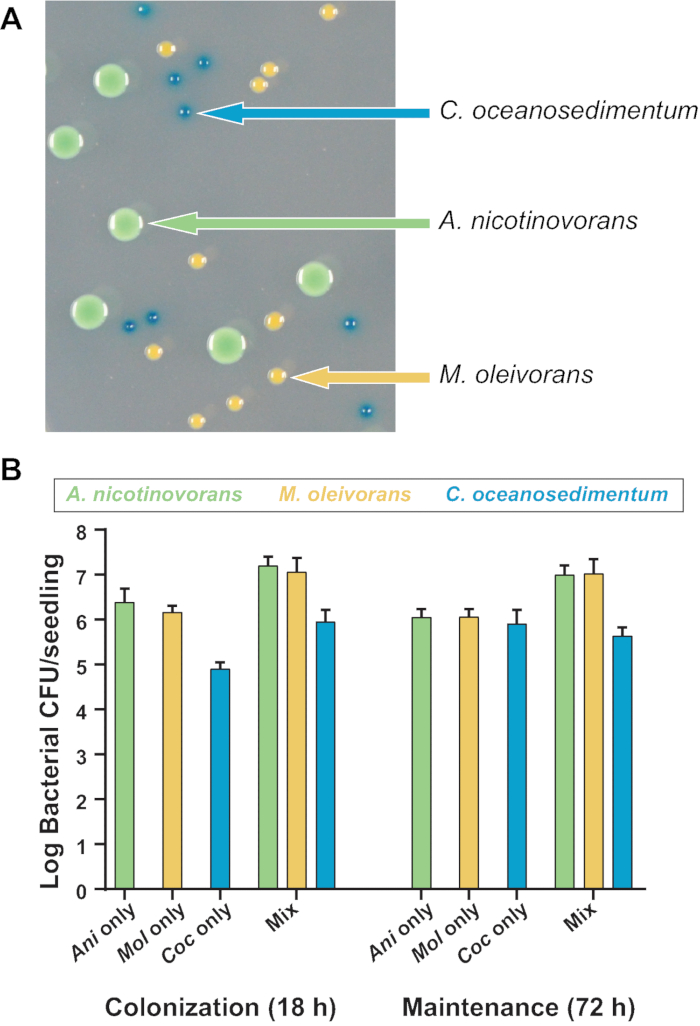
Figure 4: Quantification of the colonization and maintenance of a mixed bacterial community on A. thaliana roots. (A) Colonies of A. nicotinovorans, M. oleivorans, and C. oceanosedimentum can be differentiated on X-gal-containing agar medium by colony morphology and color. (B) Roots of 10 day-old seedlings were inoculated with approximately 3×105 CFU/mL of each of the three strains. Shown are total CFU/seedling recovered of each species following 18 h of colonization or 72 h of maintenance when colonized either alone or in a three-member bacterial community. Two biological replicates, each comprising two technical replicates of two seedlings per float, are shown. The numbers shown are the means from the two technical replicates, while bars represent standard errors of the means . Please click here to view a larger version of this figure.
Supplemental Figure 1: Ultrasonication disrupts the root surface. To dislodge bacteria from the surface of the root, whole plants were sonicated, and the bacteria was released into the liquid, which was serially diluted and plated for quantification of CFU/seedling. (A) An intact seedling is (B) structurally disrupted following ultrasonication. Please click here to download this file.
Discussion
Plants in all environments interact with thousands to millions of different bacteria and fungi5,7. These interactions can either negatively and positively impact plant health, with potential effects on crop yield and food production. Recent work also suggests that variable colonization of crops by PGPBs may account for unpredictable plant size and crop yield in field trials22. Understanding the mechanisms behind these interactions might allow us to directly manipulate plant-associated microbial communities to aid in healthy plant development, even under stress24.
Because bacterial colonization of roots and their maintenance in the rhizosphere is critical for plant-microbe interactions9, we wanted to build a system to reproducibly visualize and quantify these bacterial behaviors. This hydroponic, floating seedling growth system allows for microscopic imaging and quantification of bacterial populations on the roots of A. thaliana.
The described plant-microbe interaction assay integrates beneficial elements of existing experimental protocols. The floating mesh method was based on that from Haney et al.13, which measured initial colonization of P. simiae WCS417r on static, floating A. thaliana seedlings. In evaluating this system, strong colonization of A. thaliana roots by P. simiae was validated, even though a different growth medium from Haney et al. was utilized and included orbital shaking during colonization. The inclusion of orbital shaking during both colonization and maintenance facilitates bacterial interactions that might not occur in static culture, as well as reduce anoxic conditions that can inhibit both bacterial growth and plant root health13. We also integrated aspects of microbiology-focused protocols designed to support plant root colonization by various bacterial species8,15,25. This included a crucial transfer step out of the medium optimized for bacterial colonization and into a medium optimized for plant growth. This transfer to fresh medium also will allow the mechanisms underlying bacterial maintenance on roots to begin to be addressed, an approach that may provide insights into the erratic maintenance of PGPB in field trials6.
This assay was optimized for rapid processing of multiple samples to allow for environmental variables and mixed bacterial communities to be assayed within one multi-well biological replicate. While ultrasonication has been previously shown to be sufficient for disruption and collection of rhizosphere bacteria, the 24-well plate and multi-prong horn attachment quickens sample processing. This cell viability calculation approach to quantifying bacterial presence could be complemented by, or expanded to include, qPCR or MiSeq 16S rRNA gene community profiling to determine the relative abundance of more diverse communities of colonizing bacteria20. In addition, during imaging, the utility of focusing on just three regions of each plant root to speed the visualization of bacterial presence and localization on the roots is highlighted. The colonization of these different root regions has been shown to differ among bacterial species14. Imaging can be performed with either naturally autofluorescent bacteria or genetically tractable bacteria engineered to express a fluorescent protein.
The methodology described here allows for fast and reproducible evaluation of root colonization by PGPB bacteria, but there are limitations to the conclusions that can be drawn from these experiments. For instance, the ability for bacteria to chemotax towards the root is known to be important for many bacterial species’ colonization of plant roots, but this process may not required within this shaking inoculation system. That said, for studies specifically interested in chemotaxis, the colonization step could be performed in static liquid culture or on the surface of a soft agar medium, where bacteria could be plated distant from the plant, requiring them to actively move towards the root. In addition, a relatively rich growth medium during colonization was used to promote bacterial growth and plant attachment; however, these comparatively high nutrient concentrations may prohibit the examination of bacterial utilization of or competition for plant-derived carbon during colonization. Again, depending on the growth requirements of the bacteria being studied, the colonization medium can be varied to best suit the particular research questions of specific researchers.
This system was designed to be easily amenable to different bacterial and plant growth conditions and to the addition of different environmental stressors and timepoints. However, the methods described here are best suited for measuring bacterial interactions with the roots of A. thaliana seedlings. Collection has been optimized for this plant, and larger or more sensitive plants may be intractable in the floating multi-well-plate system. Finally, while the bacteria of interest used here colonize the plant root in liquid culture, for other bacteria it may be necessary to inoculate the plant roots by dip-inoculation or plating on a solid agar medium instead19.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by research funds provided by the Department of Energy Biological and Environmental Research (DOE-BER 0000217519 to E.A.S.), the National Science Foundation (INSPIRE IOS-1343020 to E.A.S). SLH was also supported by the National Science Foundation Graduate Research Fellowship Program. We thank Dr. Jeffery Dangl for providing bacterial strains and invaluable insight. We thank Dr. Andrew Klein and Matthew J. Powers for experimental suggestions. Finally, SLH would like to thank connections on social media for reminding us that disseminating science is a privilege and a responsibility, especially through creative and accessible means.
Materials
| Required Materials | |||
| 1.5 mL eppendorf tubes | any | N/A | |
| 24-well plates | BD Falcon | 1801343 | |
| Aeraseal | Excel Scientific | BE255A2 | |
| Autoclave | any | N/A | |
| Bacteria of Interest | any | N/A | Stored at -80˚C in 40% glycerol preferred |
| BactoAgar | BD | 2306428; REF 214010 | |
| bleach | any | N/A | |
| Conviron | any | N/A | Short Day Light-Dark Cycles: 460-600 µmoles/m²/s set at 9/15 hours light/dark at 18/21˚C, with inner power outlet |
| Dessicator Jar: glass or heavy plastic | any | N/A | |
| Ethanol | any | N/A | |
| Flame | any | N/A | |
| Forceps | any | N/A | |
| Incubator | any | N/A | At optimal temperature for growth of specified bacteria |
| Hydrochloric Acid | any | N/A | |
| Lennox LB Broth | RPI | L24066-1000.0 | |
| Microcentrifuge | any | N/A | |
| Micropipetters | any | N/A | Volumes 5 µL to 1000 µL |
| Microscope (preferably fluorescence) | any | N/A | Could be light if best definition not important |
| MS Salts + MES | RPI | M70300-50.0 | |
| Orbital Plate Shaker | any | N/A | Capable of running at 220 rpm for at least 96 hours |
| Petri Dishes | any | N/A | 50 mL total volume |
| Reservoirs | any | N/A | |
| Spectrophotometer | any | N/A | |
| Standard Hole Punch | any | N/A | Approximately 7mm punch diameter |
| Sterile water | any | N/A | |
| Surgical Tape | 3M | MMM1538-1 | |
| Teflon Mesh | McMaster-Carr | 1100t41 | |
| Ultrasonicator | any | N/A | |
| Vortex Mixer | any | N/A | |
| X-gal | GoldBio | x4281c | other vendors available |
| Suggested Materials | |||
| 24 Prong Ultrasonicator attachment | any | N/A | For sonicating multiple samples at once. Can be done individually |
| Alumaseal II | Excel Scientific | FE124F | |
| Glass beads | any | N/A | |
| Multipetter/Repetter | any | N/A | |
| Sterile 96-well plates | any | N/A | For serial dilutions. Can be replaced by eppendorf tubes |
| Biological Materials Used | |||
| Arabidopsis thaliana seeds | any | N/A | We recommend Arabidopsis Biological Resource Center for seed stocks |
| Arthrobacter nicotinovorans | Levy, et al. 2018 | ||
| Curtobacterium oceanosedimentum | Levy, et al. 2018 | ||
| Microbacterium oleivorans | Levy, et al. 2018 | ||
| Pseudomonas simiae WCS417r | Published in a similar system in Haney, et al. 2015. Strain used developed in Cole, et al. 2017 |
References
- Strange, R. N., Scott, P. R. Plant disease: a threat to global food security. Annual Review of Phytopathology. 43, 83-116 (2005).
- Cook, A. M., Grossenbacher, H., Hütter, R. Isolation and cultivation of microbes with biodegradative potential. Experientia. 39 (11), 1191-1198 (1983).
- Vacheron, J., et al. Plant growth-promoting rhizobacteria and root system functioning. Fronteirs in Plant Science. 4, 356 (2013).
- Backer, R., et al. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Fronteirs in Plant Science. 9, 1473 (2018).
- Zamioudis, C., Pieterse, C. M. Modulation of host immunity by beneficial microbes. Molecular Plant-Microbe Interactions. 25 (2), 139-150 (2012).
- Kröber, M., et al. Effect of the strain Bacillus amyloliquefaciens FZB42 on the microbial community in the rhizosphere of lettuce under field conditions analyzed by whole metagenome sequencing. Frontiers in Microbiology. 5, 252 (2014).
- Bulgarelli, D., Schlaeppi, K., Spaepen, S., Ver Loren van Themaat, E., Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annual Review of Plant Biology. 64, 807-838 (2013).
- Niu, B., Paulson, J. N., Zheng, X., Kolter, R. Simplified and representative bacterial community of maize roots. Proceedings of the National Academy of Sciences USA. 114 (12), E2450-E2459 (2017).
- Richter-Heitmann, T., Eickhorst, T., Knauth, S., Friedrich, M. W., Schmidt, H. Evaluation of Strategies to Separate Root-Associated Microbial Communities: A Crucial Choice in Rhizobiome Research. Frontiers in Microbiology. 7, 773 (2016).
- Shank, E. A. Using coculture to detect chemically mediated interspecies interactions. Journal of Visual Experiments. (80), e50863 (2013).
- Woodward, A. W., Bartel, B. Biology in Bloom: A Primer on the Arabidopsis thaliana Model System. Genetics. 208 (4), 1337-1349 (2018).
- Alatorre-Cobos, F., et al. An improved, low-cost, hydroponic system for growing Arabidopsis and other plant species under aseptic conditions. BMC Plant Biology. 14, 69 (2014).
- Haney, C. H., Samuel, B. S., Bush, J., Ausubel, F. M. Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nature Plants. 1 (6), (2015).
- Massalha, H., Korenblum, E., Malitsky, S., Shapiro, O. H., Aharoni, A. Live imaging of root-bacteria interactions in a microfluidics setup. Proceedings of the National Academy of Sciences USA. 114 (17), 4549-4554 (2017).
- Townsley, L., Yannarell, S. M., Huynh, T. N., Woodward, J. J., Shank, E. A. Cyclic di-AMP Acts as an Extracellular Signal That Impacts. MBio. 9 (2), (2018).
- Beauregard, P. B., Chai, Y., Vlamakis, H., Losick, R., Kolter, R. Bacillus subtilis biofilm induction by plant polysaccharides. Proceedings of the National Academy of Sciences U S A. 110 (17), E1621-E1630 (2013).
- Matthysse, A. G. Adherence of Bacteria to Plant Surfaces Measured in the Laboratory. Journal of Visual Experiments. 136 (136), (2018).
- Garcia-Betancur, J. C., Yepes, A., Schneider, J., Lopez, D. Single-cell analysis of Bacillus subtilis biofilms using fluorescence microscopy and flow cytometry. Journal of Visual Experiments. (60), (2012).
- Cole, B. J., et al. Genome-wide identification of bacterial plant colonization genes. PLoS Biology. 15 (9), e2002860 (2017).
- Lundberg, D. S., et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 488 (7409), 86-90 (2012).
- Grandchamp, G. M., Caro, L., Shank, E. A. Pirated Siderophores Promote Sporulation in Bacillus subtilis. Applied Environmental Microbiology. 83 (10), (2017).
- Gange, A. C., Gadhave, K. R. Plant growth-promoting rhizobacteria promote plant size inequality. Science Reports. 8 (1), 13828 (2018).
- Levy, A., et al. Genomic features of bacterial adaptation to plants. Nature Genetics. 50 (1), 138-150 (2018).
- Martínez-Hidalgo, P., Maymon, M., Pule-Meulenberg, F., Hirsch, A. M. Engineering root microbiomes for healthier crops and soils using beneficial, environmentally safe bacteria. Canada Journal of Microbiology. , 1-14 (2018).
- Niu, B., Kolter, R. Quantification of the Composition Dynamics of a Maize Root-associated Simplified Bacterial Community and Evaluation of Its Biological Control Effect. Bio Protocol. 8 (12), (2018).

