Performing In Situ Closed-Cell Gas Reactions in the Transmission Electron Microscope
Summary
Here, we present a protocol for performing in situ TEM closed-cell gas reaction experiments while detailing several commonly used sample preparation methods.
Abstract
Gas reactions studied by in situ electron microscopy can be used to capture the real-time morphological and microchemical transformations of materials at length scales down to the atomic level. In situ closed-cell gas reaction (CCGR) studies performed using (scanning) transmission electron microscopy (STEM) can separate and identify localized dynamic reactions, which are extremely challenging to capture using other characterization techniques. For these experiments, we used a CCGR holder that utilizes microelectromechanical systems (MEMS)-based heating microchips (hereafter referred to as "E-chips"). The experimental protocol described here details the method for performing in situ gas reactions in dry and wet gases in an aberration-corrected STEM. This method finds relevance in many different materials systems, such as catalysis and high-temperature oxidation of structural materials at atmospheric pressure and in the presence of various gases with or without water vapor. Here, several sample preparation methods are described for various material form factors. During the reaction, mass spectra obtained with a residual gas analyzer (RGA) system with and without water vapor further validates gas exposure conditions during reactions. Integrating an RGA with an in situ CCGR-STEM system can, therefore, provide critical insight to correlate gas composition with the dynamic surface evolution of materials during reactions. In situ/operando studies using this approach allow for detailed investigation of the fundamental reaction mechanisms and kinetics that occur at specific environmental conditions (time, temperature, gas, pressure), in real-time, and at high spatial resolution.
Introduction
There is a need to obtain detailed information on how a material undergoes structural and chemical changes under reactive gas exposure and at elevated temperatures. In situ closed-cell gas reaction (CCGR) scanning transmission electron microscopy (STEM) was developed specifically to study the dynamic changes occurring in a wide range of material systems (e.g., catalysts, structural materials, carbon nanotubes, etc.) when subjected to elevated temperatures, different gaseous environments, and pressures from vacuum to full atmospheric pressure1,2,3,4,5,6,7,8,9,10,11,12. This approach can be beneficial in several cases, e.g., in the accelerated development of next-generation catalysts that are important for a number of industrial conversion processes, such as the single-step conversion of ethanol to n-butenes over Ag-ZrO2/SiO213, catalysts for the oxygen reduction reaction and hydrogen evolution reaction in fuel cell applications14,15, catalytic CO2 hydrogenation16, methanol dehydrogenation to formaldehyde or dehydration to dimethyl ether that use either metal catalysts or multi-walled carbon nanotubes in a methanol conversion reaction in the presence of oxygen17. Recent applications of this in situ technique for catalysis research1,2,7,8,10,11,12,18,19,20,21,22 have provided new insight into catalyst dynamic shape changes10,11,23, faceting7, growth, and mobility8,20,24. Moreover, in situ CCGR-STEM can be used to investigate the high-temperature oxidation behavior of structural materials that are exposed to aggressive environments, from gas turbine engines to next-generation fission and fusion reactors, where not only strength, fracture toughness, weldability, or radiation are important but also high-temperature oxidation resistance25,26,27,28,29. Specific to structural alloys, in situ CCGR-STEM experiments allow for dynamic tracking of diffusion-induced grain boundary migration under reducing conditions9 and measurements of oxidation kinetics at high temperature5,6,30. For several decades prior to the recent development of CCGR technologies, in situ gas reaction studies were conducted using dedicated environmental TEMs (E-TEMs). A detailed comparison of E-TEM and CCGR-STEM has been previously addressed10; therefore, E-TEM capabilities are not discussed further in the present work.
In this work, a commercially available system (Table of Materials) comprising a computer-controlled manifold (gas delivery system) and a specially designed CCGR TEM holder that utilizes a pair of microelectromechanical (MEMS)-based silicon microchip devices (e.g., spacer chip and "E-chip" heater (Table of Materials)) were used. Each E-chip supports an amorphous, electron-transparent SixNy membrane. The spacer chip has a 50 nm thick SixNy membrane with a 300 x 300 µm2 viewing area and 5 µm thick epoxy-based photoresist (SU-8) "spacer" contacts that are microfabricated to provide a gas flow path and maintain a physical offset between the two paired microchips (Figure 1A). A portion of the E-chip is covered with a low conductivity ~100 nm SiC ceramic membrane; the membrane has a 3 x 2 array of 8 µm-diameter etched holes overlapped by a ~30 nm thick amorphous SixNy membrane (SixNy viewing area) (Figure 1A and Figure 2D), through which images are recorded. The E-chip serves a dual role as both specimen support and heater6. Au contacts are microfabricated onto the E-chip to allow for resistive heating of the SiC membrane. Each E-chip is calibrated using infrared radiation (IR) imaging methods (Table of Materials)2 and has been shown to be accurate to within ±5%31. Temperature calibration is independent of the gas composition and pressure, thereby providing independent control over reaction temperatures under any chosen gas conditions. The benefit of a thin-film heater is that temperatures up to 1,000 °C can be reached within milliseconds. In order to perform the reaction, the E-chip is placed on the top of the spacer chip, creating the closed-cell "sandwich" that isolates the environment around the specimen from the high vacuum of the TEM column. The advantage of this setup is that reactions can be performed from low pressures up to atmospheric pressure (760 Torr) with single or mixed gases and under static or flow conditions. The MEMS devices are secured with a clamp (Figure 1B) that allows the holder to be inserted within the mm-sized gap of the objective lens pole piece in an aberration-corrected S/TEM instrument (Table of Materials) (Figure 1C). Modern in situ S/TEM holders include integrated micro-fluidic tubing (capillaries) that are connected to the external stainless-steel tubing, which in turn is connected to the gas delivery system (manifold). An electronic control system permits the controlled delivery and flow of reactant gas through the gas cell. Gas flow and temperature are operated by a custom workflow-based software package provided by the manufacturer (Table of Materials)10,32. The software controls three gas input lines, two internal experimental-gas delivery tanks, and a receiving tank for gas flow returning from the cell during the experiment (Figure 1D).
Due to the variability of materials and their form factor, we first focus on several specimen deposition methods on the E-chip, then outline protocols for performing quantitative in situ/operando experiments with controlled temperature, gas mixing and flow.
Protocol
1. E-chip preparation
- Direct powder deposition by drop-casting from a colloidal solution (Figure 2A).
- Crush the powder if the powder particle aggregates are too large. Do this using a small mortar and pestle (crushed aggregates should be <5 µm in size). Mix a small amount (e.g., ~0.005 mg, amount determined by experience) of powder in 2 mL of the solvent (e.g., isopropanol or ethanol).
- Sonicate the mixture for around 5 min to create a colloidal suspension.
- Place the E-chip on the E-chip retaining fixture. Drop cast approximately 1 µL of the suspension using a 0.5-2.5 µL micro-pipette directly onto the E-chip.
- Clean the Au contacts to remove the suspension with an absorbent paper point while viewing through a stereo microscope.
- Direct powder deposition through a mask (Figure 2B).
- Crush the powder (e.g., Pt/TiO2) dry, if powder particles are too large (as in 1.1.1).
- Place a new clean E-chip on the E-chip retaining fixture (Figure 3D). Use a mask, which is another E-chip with the SixNy membrane removed (by breaking it with tweezers or compressed gas) and place it directly onto the E-chip within the fixture.
- Use the top plate to clamp a new clean E-chip and a mask together within the fixture.
- Deposit a small amount of the powder using a spatula directly on the silicon nitrile membrane in the mask.
- Gently vibrate the fixture to shake the particles down to the E-chip. This can either be done using a vacuum tweezer unit by holding the fixture to the top of the unit while it is running or using a sonication unit and placing the fixture in a dry beaker.
- Shake off the excess powder, disassemble the system and inspect the placement of dry powder on the E-chip using a stereo microscope.
- Deposition method by either electron beam evaporation, ion, or magnetron sputtering.
NOTE: This method is used to create either a single-element system or model alloy specimens of known geometry and composition.- Create a pattern mask (Figure 3).
NOTE: Prepare the pattern mask in advance since it takes some time. - Use a spacer chip with removed SixNy membrane. In this experiment, an E-chip commonly used in liquid-cell experiments was used after gently breaking out the SixNy membrane which resulted in 50 x 250 µm opening. This spacer chip with removed SixNy membrane will be combined with another chip, having an array of holes (e.g., silicon nitride (SiN) Microporous TEM Window 33).
- Use cyanoacrylate (CA) glue (Table of Materials) to attach the SiN Microporous TEM Window face down (SiN pattern film away from the spacer chip) over the 50 x 250 µm opening following the manufacturer's recommendation (Figure 3B,C).
- Repeat the procedure to prepare as many pattern masks as needed, depending on the planned experiments.
- Place a new clean E-chip on the E-chip fixture (Figure 3D).
- Place the pattern mask on the E-chip (Figure 3C,D).
- Cover with the top plate and clamp it (Figure 3D).
- Use either electron beam evaporation, ion sputtering or magnetron sputtering deposition techniques. These are the recommended methods used to sputter material of interest directly through the pattern mask.
NOTE: It may be important to purge the deposition system to remove residual oxygen prior to the deposition for higher purity material deposits33. - Disassemble the system and inspect the E-chip with a stereo microscope to ensure good adherence of the deposited material on the E-chip's SixNy membrane.
- Create a pattern mask (Figure 3).
- Focused ion beam (FIB) milling (Figure 2C).
- Prepare a standard TEM lamella using the FIB. Use low kV (e.g., 2-5 kV) for the final milling step to remove damage caused by FIB milling at high voltages (30-40 kV).
- Place the TEM lamella on the E-chip using standard FIB procedures. Do not damage the SixNy membrane when attaching the FIB-prepared TEM lamella to the E-chip. See Allard et al.34 and other publications30,35,36 for details of the variety of methods using Xe-PFIB and Ga-FIB instruments for lamella preparation.
2. Preparation of the atmosphere (CCGR-TEM) holder
- Download the desired calibration file.
- Measure the resistance of the SiC heater to ensure that it is within the resistance range for that particular E-chip calibration as provided by the CCGR manufacturer.
- Remove the clamp from the CCGR-TEM holder.
- Clean the tip of the CCGR-TEM holder using absorbent paper points and/or compressed air, making sure no debris remains on the O-ring grooves. Then place the special double-gasket seal within the tip.
- Place the spacer chip into the CCGR-TEM holder.
- Place the E-chip containing the specimen that was prepared by one of the methods mentioned in section 1 with the heater contacts down onto the spacer chip, making a proper connection to the electrical contacts of the flex-cable within the holder.
- Position the holder clamp plate on the top of the E-chip using tweezers, place the screws into the designated location at the tip of the CCGR-TEM holder, then torque the set screws with a final torque to 0.2 lb-ft.
- Measure again, the resistance of the SiC heater after assembling the CCGR-TEM holder to ensure that it is within the resistance range for that particular E-chip calibration as provided by the CCGR manufacturer.
NOTE: Here, a special adapter is used, which plugs directly into the holder's electrical connections. This allows for the resistance measurements to be made through the CCGR-TEM holder and paired microchip devices assembly while fully assembled into the holder.
3. Preparation of the experimental setup
- Bake and pump down the system (manifold, holder, gas tanks, and RGA chamber) overnight, either with or without the holder connected by pressing the Bake button in the gas-control software.
- Load the holder into the scanning transmission electron microscope and connect the gas tubing from the manifold to the CCGR-TEM holder.
- For the experiment, pump and purge the system with an inert gas (e.g., Ar or N2) twice from 100 Torr to 0.5 Torr.
- Perform a final pump and purge from 100 Torr to 0.001 Torr. This will ensure that the entire gas delivery system, from the gas manifold to the holder, is cleaned and flushed with inert gas.
- Residual gas analyzer – During the pump and purge procedure, turn on the RGA system to warm up the filament.
4. Preparing the water vapor delivery system (VDS)
NOTE: These instructions are for specific experiments that involve controlled delivery of gas in vapor form (e.g., water vapor). Gas delivery control is through the gas-control software provided by the manufacturer (Table of Materials).
- Attach the purge gas (e.g., N2) to the VDS, turn the lever knob to Exhaust, and then turn to the Park position.
- Purge the VDS (repeat 4.1) by flowing inert gas three times or until no more liquid is present.
- Turn the lever knob to the Park position and attach the VDS to the manifold.
- Turn the lever knob to the Fill position and remove the purge gas line.
- Set the vapor pressure to 18.7 Torr in the gas-control software.
- In the software, pump the VDS to vacuum (0.1 Torr) by selecting the input line and pressing the pump button.
- Fill the VDS with water (2 mL) via a syringe and tubing.
NOTE: If higher purity vapor is needed, additional purging steps may be required.
5. Running the reaction
- Make sure all gases that are to be used in the experiments (e.g., N2, water vapor, and O2) are connected to the manifold.
- With the gas-control software under Naming, set the name(s) for the gas(es) required for the reaction and save the raw ".csv" file such that a running log file is generated for the experiment.
- Under the E-chip Setup, select the associated calibration file (i.e., as described in 2.5) for the E-chip being used and Run Calibration. As previously mentioned in the Introduction section, each E-chip is temperature calibrated using infrared radiation (IR) imaging from the manufacturer.
- Under Pump and Purge, see Preparation of Experimental Setup.
- Under Gas Control, select the desired gas name and its composition (e.g., select percentage for each gas) for the experiment.
- Under Temperature, select the desired heating rate and target temperature for the temperature of interest for the experiment and press the Start button.
- Start flowing the gas by pressing the Start button under the Gas Control section.
6. End of the experiment
- Once the reaction is complete, stop flowing the gas, turn off the temperature knob, and end the session using the Pump and Purge procedure (e.g., depending on the reaction that was performed, perform Pump and Purge procedure from 100 Torr to 0.1 Torr 2-3 times).
- Prior to removing the in situ CCGR-TEM holder from the electron microscope, ensure that that holder pressure is brought back up to atmospheric pressure.
Representative Results
Specimens for MEMS-Based Closed-Cell Gas Reactions:
Direct powder deposition by drop casting from a colloidal solution and through a mask
Depending on the material to be studied, there are a number of different ways to prepare E-chips for in situ/operando CCGR-STEM experiments. Preparing the gas cell for catalysis studies typically requires dispersion of the catalyst nanoparticles onto the E-chip either from a colloidal liquid suspension (Figure 2A), or directly from the dry powder itself (Figure 2B). For coarser powders, it may be necessary to crush the particles (e.g., using a mortar and pestle or by placing the powder between glass slides), so the powder aggregates will fit within the 5 µm gap between paired microchips ("sandwich") size without damaging the SixNy membranes. When using a liquid suspension, the deposition of powders results in wider dispersion covering a larger area of the E-chip, which often requires a secondary cleaning ("dusting") step to remove the powder from the gold contacts. Whereas, when depositing dry powder, a mask can be used to directly deposit powder in the desired location (e.g., the electron-transparent SixNy viewing area). In our study, the masks we tested are E-chips with removed SixNy membrane and liquid-cell spacer chip with removed SixNy film. Since the latter one has a narrower opening (50 x 250 µm), a more precise deposition can be achieved directly onto the membrane heater region of the E-chip, and no additional cleaning of the gold contacts is necessary.
Pattern mask and alloy deposition
Deposition of the catalyst on the E-chip is relatively easy when compared with bulk alloys. Since nano-sized particles of random alloy compositions are not readily available and crushing alloy micro-size powders has also been problematic6, the evaluation of one more potential new method was addressed for producing alloy specimens of controlled composition and geometry onto gas-cell E-chip membranes33.
The basic idea for the structural alloy specimens is to deposit "islands" (Figure 2D) of the desired structural material using a suitable vapor deposition technique (e.g., electron beam evaporation, ion sputtering, or magnetron sputtering) where the elemental species are deposited directly onto the E-chip membrane (Figure 3A) through a pattern mask composed of an array of ~2 µm diameter holes (Figure 3). The pattern mask can be produced by FIB-milling techniques, using a SixNy spacer E-chip. Alternatively, it is easier to use a commercially available 50 nm thick SiN Microporous TEM Window, with an array of 2 µm pores in a silicon nitride film in a grid pattern within a single 500 x 500 µm membrane (Table of Materials)33 as a pattern mask (Figure 3B-b). As shown, it is possible to attach a SiN Microporous TEM Window to an E-chip with removed SixNy membrane (Figure 3B-a) and place it directly on the E-chip (Figure 3C) in a securely tightened E-chip fixture (Figure 3D). This is used to create a perfect alignment of the devices and helps isolate the evaporated species to a small area on the E-chip (Figure 3C-c and 3C-d). Depending on the chemical composition of the depositing alloy/material, each evaporation technique (electron beam evaporation, ion sputtering, or electron magnetron sputtering) has its own advantages and disadvantages33, which will not be addressed here. Therefore, the idea for gas-reactor sample preparation by the vapor-phase deposition through a pattern mask onto the E-chip surface has potential for further development and experimentation.
FIB milling
E-chip preparation becomes more challenging when investigating solid materials. Comparable studies of bulk structural materials require preparation of the sample as a thin slice or lamella of suitable specimen thickness and geometry (e.g., electron transparent, and a few microns in lateral extent) that can be secured in some fashion to the E-chip membrane. This process can be conducted using FIB-milling procedures and placing the TEM lamella on the SixNy viewing area in the SiC heater membrane (Figure 2C-c)9,30,36,37 with the caveat that conventional gallium FIB milling typically leaves residual Ga, either as Ga implantation and/or Ga segregation in some material systems (e.g., within grain boundaries and phases in Al and its alloys38) on the milled surface, thereby complicating the reaction process when dynamic events need to be examined at the atomic level. It is essential to evaluate material susceptibility to Ga penetration9. To minimize Ga implantation and surface damage, we can use electropolished needles, similar to the ones used for atom probe tomography, which can then be placed on the E-chip (Figure 2C-d) using the FIB by attaching the sample by W or Pt "tack" points31. EDS analysis confirms that Ga implantation can be decreased/eliminated (Figure 2C-d); however, the limitation of this method is the geometry of the sample. Only needle-shaped samples can be prepared without exposing the area of interest to Ga ions. As an alternative, new Xe-plasma FIBs can be used to prepare thin lamella without Ga implantation. For example, electron-transparent lamella from 3-mm electropolished discs can be extracted and placed on the E-chip (Figure 2C-e) resulting in a large area of the sample with no issues associated with a residual ion implantation layer (Xe is inert and does not tend to deposit on sample surfaces. It also produces a thinner amorphous layer (~1 nm) than the best FIB procedures with a Ga source)34.
In situ reaction experiments
In order to capture dynamic events, first, it is necessary to bake and pump down the system overnight. During the actual experiment, the holder is connected to the gas manifold system and is pumped and purged several times. The system is initially pumped down twice from 100 Torr to 0.5 Torr and purged with an inert gas (e.g., N2, Ar); the third cycle involves pumping down to 0.001 Torr. The internal conditions are monitored by an RGA system (Table of Materials), which is equipped with an electron multiplier10. The RGA is integrated into the gas-control system through connection to the return side of the CCGR-TEM holder (Figure 4B). To remove residual water vapor and other gases from the RGA chamber, heating tape is used which permits for bake-outs between experiments. An ultra-high vacuum in the RGA of < 2×10-8 Torr can be achieved. An electronically controlled leak valve (LV) is used to control the amount of gas from the holder and into the RGA chamber, and a return capillary line to the manifold is isolated from the leak valve with a hand valve (HV).
An example of the recorded gas partial pressures measured in the RGA chamber, CCGR-TEM holder (LV open), manifold (H1 open), and Tank 1 (T1 open) before in situ experiments is shown in Figure 4. This demonstrates that even though overnight baking, pumping down and purging were performed, there is still some degree of residual water vapor. Thus, for experiments particularly with water vapor, it is important to establish the baseline for initial conditions of the system and record the initial partial pressures. For our system shown in Figure 4, the partial pressure of water vapor measured all the way to Tank 1 reads 1.1 x 10-7 Torr. The atomic mass spectrum versus partial pressure shows the water vapor peak at 18 amu, reaching 1.1 x 10-7 Torr (Figure 3C). Comparing the spectrum with the one from the experiments that contained O2 and water vapor, there is a significant increase in the partial pressure (2.5 x 10-7 Torr) of the peak at 18 amu. Note that by further opening the leak valve, more gas flow is introduced into RGA chamber where measurements are performed. It is important to adjust the leak valve in such a way that the total pressure of the experiment is kept constant in order to compare results between conditions. Gas composition measurements are possible when the RGA chamber pressure is in the ≤10-5 Torr range, which is less than a billionth of an atmosphere due to the high reactivity of ions and their short life; therefore, the pressures in the RGA are much lower than within the gas cell.
The attachment of the water vapor delivery system to the manifold requires purging the VDS with inert gas until no liquid is present (it is also important to clean the VDS right after the experiment to make this step simpler) and keep it purged during the connection to the manifold. Before the VDS is filled with the desired liquid (e.g., water, methanol, or ethanol), first, the VDS is pumped down to vacuum. Then, the liquid is added using a syringe and tubing. To improve the quality of the vapor (with reduced oxygen content) the experimental supply tank can be filled with the vapor and pumped down two or three times; otherwise, it is ready to be used.
The gas-control software guides the user through the settings during all phases of the experiment. In the beginning, the correct gases and pressures need to be selected. The resistance of the E-chip must be checked to ensure that the E-chip was not damaged during loading into the CCGR-TEM holder. In the manifold, there are two supply tanks (Tank 1 and Tank 2) that hold and supply gas with a final composition for the reaction. The desired gas composition can be obtained by mixing the media directly in one of the supply tanks (Tank 1 or Tank 2 in Figure 1D and 4B). The manifold system has three ports that introduce gases to the manifold. However, if more than three gases are desired to be mixed, one or more of the input lines need to be split. Alternatively, if the gas composition is very complicated, pre-mixed gases should be used, which allows their mixing during the experiment with the desired vapor composition.
After setting the desired gas composition for the in situ experiment, the gas-control software will first introduce the lower percentage gas; then after reaching the desired pressure, it will feed the second gas into the supply tank. Afterward, depending on the experiment, the gas can be introduced into the gas cell at either room temperature or after heating the sample to the desired temperature at a certain/desired heating rate. This depends on each user's experiment. The heating can occur in a vacuum, under inert gas, or under the premixed gas that will be used in the experiments. When the gas needs to be changed while running experiments, the system is pumped down and purged with inert gas to avoid any hazard of mixing two incompatible gases.
In general, there is little or no drift in the x and y directions during the experiments, but during heating and/or pressure changes, significant variation in the specimen height is observed (which poses a challenge to capture the initiation of a reaction). If possible, heat to the desired temperature under vacuum or inert gas, adjust all the alignments and then introduce the gas media. Experiments below 200 °C are also challenging with the closed-cell due to contamination build up on the surface of the E-chip viewing area.
As an example, the evolution of the surface of Pt nanoparticles on a TiO2 support was captured when exposed to 100% water vapor at 17 Torr at 300 °C (Figure 5). The structural changes in the Pt particle and rearrangement of the structure to expose {111} surfaces (Figure 6) were observed (Figure 6A vs. Figure 6B vs. Figure 6C).
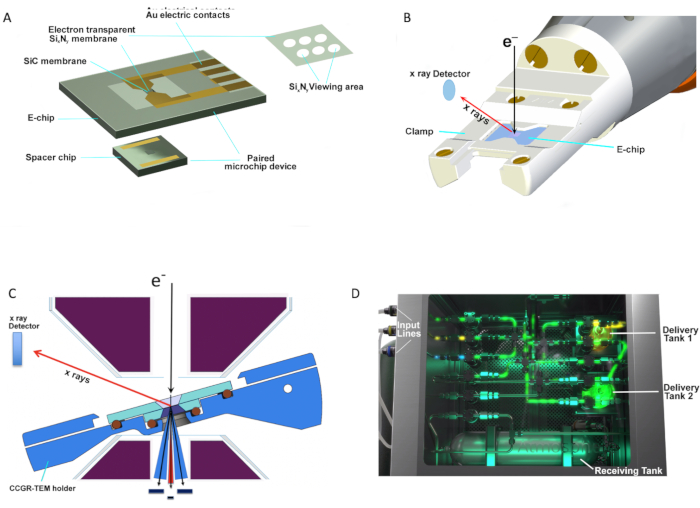
Figure 1: E-chip paired device with CCGR-TEM holder. (A) Pair of MEMS-based silicon microchip devices (spacer chip and E-chip (heater)) for in situ CCGR-STEM experiments. (B) Schematic of the CCGR-TEM holder tip with paired microchip devices being secured with a clamp. (C) Cross-section of the CCGR-TEM holder tip showing E-chip placed on top of the spacer chip creating the closed-cell (sandwich) that isolates environment around the specimen from TEM column. (D) Closer view of manifold that encloses three gas input lines on the side, two experimental gas delivery tanks, and a receiving tank for gas flow control during the experiment. (Images provided by the CCGR system manufacturer). Please click here to view a larger version of this figure.

Figure 2: Example of different deposition techniques for preparing samples on the E-chip. (A) E-chip with catalyst deposited by drop-casting from a colloidal solution. (B) E-chip after dry powder deposition using two different masks (a) E-chip with removed SixNy membrane and (b) liquid-cell E-chip with removed SixNy membrane. (C) E-chip prepared by (a) standard FIB-milling procedures and placement of FIB lamella on SixNy electron transparent viewing areas, (b) electropolished needle, (c) electron-transparent section of a grain in a 3 mm electropolished disc extracted by Xe-plasma FIB and placed on E-chip. (D) Higher magnification image of E-chip with alloy deposited through pattern mask. Please click here to view a larger version of this figure.

Figure 3: E-chip preparation using sputtering techniques. (A) Schematic of the E-chip. (B) Pattern mask fabricated from liquid-cell E-chip with SixNy membrane removed and 50 nm thick SiN Microporous TEM Window (B-a), with arrays of 2 µm pores in silicon nitride film in a grid pattern within single 500 x 500 µm membrane window 34 that overlaps the 50 x 250 micron opening of the liquid-cell microchip (B-b). (C) Pattern mask directly placed on the E-chip (C-c) with higher magnification image showing the alignment of SixNy viewing area with 50 x 250 µm opening in liquid-cell E-chip that is covered with SiN Microporous TEM Window (3C-d and also 3B-b). (D) E-chip cross-section within the fixture (D-e), top view (D-g), and (D-f) closeup view of the pattern mask in the E-chip fixture. E-chip fixture holds pattern mask placed on E-chip in secure manner during vapor-phase deposition. Please click here to view a larger version of this figure.

Figure 4: Measuring gas compositions using a residual gas analyzer. (A) Example of gas partial pressures measured in RGA chamber, CCGR-TEM holder (LV open), manifold (H1 open), and Tank 1 (T1 open) before in situ experiments. (B) Schematic of the gas-control software showing locations for RGA measurements before experiments. (C) Mass spectra generated in a vacuum before the experiment (red) with water vapor peak at 18 amu reaching 1.1 x 10-7 Torr and during the experiment (blue) with a mixture of O2 with water vapor showing an increase in the partial pressure for OH, H2O, and O2. RGA confirms the presence of water vapor in in situ closed-cell. Please click here to view a larger version of this figure.

Figure 5: Measuring water vapor content. (A) Schematic of the gas-control software showing an example of 100% water vapor introduced to Tank 1 with test parameters recorded by the gas-control system at room temperature before the experiment. (B) Gas partial pressure spectra acquired using the RGA before (red) and during (blue) reaction with 100 % water vapor at 17 Torr and 300 °C. Please click here to view a larger version of this figure.

Figure 6: Experimental results of water vapor exposure effects on Pt nanoparticle structure. (A-C) BF-STEM images showing the reconstructed surface of a Pt nanoparticle on TiO2 support when exposed to 100 % water vapor at 17 Torr and 300 °C. Please click here to view a larger version of this figure.
Discussion
In the present work, an approach to perform in situ STEM reactions with and without water vapor is demonstrated. The critical step within the protocol is E-chip preparation and maintaining its integrity during the loading procedure. The limitation of the technique is (a) the specimen size and its geometry to fit the nominal 5-µm gap between paired (MEMS)-based silicon microchip devices as well as (b) a total pressure used in the experiments with water vapor since the highest total pressure depends on the quantity of water vapor6. The significance of this method with respect to existing methods is that we can perform operando experiments, i.e., we analyze the specimens under real conditions, enabled by an RGA system that confirms/monitors experimental conditions. Additionally, there are opportunities for future applications of the technique to diverse materials systems that may require different methods and procedures for sample deposition on E-chip heaters.
The E-chip preparation is shown in Figure 2, which highlights four different sample preparation methods; (1) direct powder deposition by drop cast from colloidal solution, (2) direct powder deposition through mask, (3) direct EBID/IBID or magnetron sputtering with patterned masks and (4) FIB milling. Powder deposition should include only powders with particles or aggregates less than 5 µm thick to fit within the nominal 5 µm gap between paired microchips in order to prevent damage to the SixNy viewing windows2,6. Researchers performing deposition through evaporation methods should adjust the parameters according to the elemental composition, temperature, and humidity and should minimize the oxygen level. Sample preparation using FIB milling requires users to be extremely careful to prevent damage to the SixNy membrane. Also, Ga implantation could alter alloy chemistry and affect surface diffusion. Regardless of what E-chip specimen method is selected, following sample deposition, examination of the E-chip using light optical microscopy and resistance measurements are required to verify the E-chip integrity before starting the in situ experiments.
This protocol for in situ CCGR-STEM studies enables new opportunities to visualize nanoscale gas reactions while they occur and under realistic conditions (temperature, pressure, and gas composition). Now, it is possible to reveal dynamic changes in the surface atoms and interfaces and to understand how the surface composition and structure may be controlled by external means7. For example, the structural changes in the Pt particle and rearrangement of its structure to expose {111} surfaces (Figure 6) were associated with minor shape changes (Figure 6A vs. Figure 6B vs. Figure 6C). Catalytic performance is determined by interfacial reactions that occur at site-specific catalyst interfaces, and in situ microscopy helped uncover gas-surface phenomena under water vapor in Pt/TiO2 catalysis research. Moreover, the experimental protocol presented here also contributes to an improved understanding of the in situ gas reaction process by monitoring the gas composition using an RGA. This is important because of the need to correlate the role of gas composition with structural and chemical changes that the material being studied undergoes as a direct effect of environmental exposure.
In summary, in situ CCGR-STEM studies can enable investigation of the deactivation or regeneration of catalyst materials via imaging and spectroscopy, and the investigation of chemical and morphological changes during gas reactions on bulk alloy materials. Such studies also allow for identification of the minimum temperature of initiation of e.g., the regeneration reaction and/or the maximum temperature for the reaction, as well as the nature of coarsening of supported metal particles from which kinetic information can be extracted. These studies provide a direct link to current computational models that predict the pathway of the reactions, but not time when it will happen, which is important for material optimization. The potential of this environmental closed-cell gas reaction protocol can be expanded to a number of different materials in conjunction with quantitative spectroscopy techniques such as electron energy-loss spectroscopy39 and energy-dispersive X-ray spectroscopy5,6 to identify chemical compositions and/or oxidation state changes. Moreover, this is just the beginning of a new capability that creates an advanced opportunity for materials characterization under a variety of realistic conditions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was primarily sponsored by the Laboratory Directed Research and Development Program of Oak Ridge National Laboratory (ORNL), managed by UT-Battelle LLC, for the U.S. Department of Energy (DOE). Part of the development to introduce water vapor into the in situ gas cell was sponsored by the U.S. DOE, Office of Energy Efficiency and Renewable Energy, Bio-Energy Technologies Office, under contract DE-AC05-00OR22725 (ORNL) with UT-Battle, LLC, and in collaboration with the Chemical Catalysis for Bioenergy (ChemCatBio) Consortium, a member of the Energy Materials Network (EMN). This work was authored in part by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the U.S. DOE under Contract No. DE-AC36-08GO28308. Part of the microscopy was conducted at the Center for Nanophase Materials Sciences (CNMS), which is a DOE Office of Science User Facility. Early development of in situ STEM capabilities was sponsored by the Propulsion Materials Program, Vehicle Technologies Office, U.S. DOE. We thank Dr. John Damiano, Protochips Inc., for useful technical discussions. The authors thank Rosemary Walker and Kase Clapp, ORNL production team, for support with movie production. The views expressed in this article do not necessarily represent the views of the DOE or the U.S. Government. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes.
Materials
| Atmosphere Clarity Software | Protochips | 6.5.14 | |
| Atmosphere Large Heating E-chips, 300 x 300 window, no spacer | Protochips | EAT-33AA-10 | microchip device |
| Atmosphere Small E-chips, 300 x 300 micron window, 5 micron SU-8 spacer | Protochips | EAB-33W-10 | microchip device |
| JEOL 2200FS | JEOL | microscope | |
| M-bond 610 | Electron Microscopy Sciences | 50410-30 | cyanoacrylate (CA) glue |
| Mikron M9103 IR camera | Micron | This is used by Protochips/ not available | |
| Protochips “Fusion” E-chips | Protochips | spacer chip with removed SixNy membrane | |
| Protochips Atmosphere 200 | Protochips | prototype | software |
| Residual Gas Analyzer R100 (RGA) | Stanford Research Systems | R100 SRS |
References
- Allard, L. F., et al. A new MEMS-based system for ultra-high-resolution imaging at elevated temperatures. Microscopy Research and Technique. 72 (3), 208-215 (2009).
- Allard, L. F., et al. Novel MEMS-based gas-cell/heating specimen holder provides advanced imaging capabilities for in situ reaction studies. Microscopy and Microanalysis. 18 (4), 656-666 (2012).
- Allard, L. F., et al. Innovative closed-cell reactor permits in situ heating and gas reactions with atomic resolution at atmospheric pressure. Microscopy and Microanalysis. 18 (2), 1118-1119 (2012).
- Allard, L. F., et al. Controlled in situ gas reaction studies of catalysts at high temperature and pressure with atomic resolution. Microscopy and Microanalysis. 20 (3), 1572-1573 (2014).
- Allard, L. F., et al. computer-controlled in situ gas reactions via a mems-based closed-cell system. Microscopy and Microanalysis. 21 (3), 97-98 (2015).
- Unocic, K. A., Shin, D., Unocic, R. R., Allard, L. F. NiAl oxidation reaction processes studied in situ using MEMS-based closed-cell gas reaction transmission electron microscopy. Oxidation of Metals. 88 (3-4), 495-508 (2017).
- Dai, S., et al. Revealing surface elemental composition and dynamic processes involved in facet-dependent oxidation of Pt3Co nanoparticles via in situ transmission electron microscopy. Nano Letters. 17 (8), 4683-4688 (2017).
- Dai, S., Zhang, S., Katz, M. B., Graham, G. W., Pan, X. In Situ observation of Rh-CaTiO3 catalysts during reduction and oxidation treatments by transmission electron microscopy. ACS Catalysis. 7 (3), 1579-1582 (2017).
- Burke, M. G., Bertali, G., Prestat, E., Scenini, F., Haigh, S. J. The application of in situ analytical transmission electron microscopy to the study of preferential intergranular oxidation in Alloy 600. Ultramicroscopy. 176, 46 (2017).
- Unocic, K. A., et al. Introducing and controlling water vapor in closed-cell in situ electron microscopy gas reactions. Microscopy and Microanalysis. 26 (2), 229-239 (2020).
- Vendelbo, S. B., et al. Visualization of oscillatory behaviour of Pt nanoparticles catalysing CO oxidation. Nature Materials. 13 (9), 884-890 (2014).
- Moliner, M., et al. Reversible transformation of Pt nanoparticles into single atoms inside high-silica chabazite zeolite. Journal of the American Chemical Society. 138 (48), 15743-15750 (2016).
- Dagle, V., et al. Single-step conversion of ethanol to n-butenes over Ag-ZrO2/SiO2 catalysts. ACS Catalyst. 10 (18), 10602-10613 (2020).
- Chi, M., et al. Surface faceting and elemental diffusion behaviour at atomic scale for alloy nanoparticles during in situ annealing. Nature Communications. 6 (1), 1-9 (2015).
- Zhao, X., et al. Single-iron site catalysts with self-assembled dual-size architecture and hierarchical porosity for proton-exchange membrane fuel cells. Applied Catalysis B: Environmental. 279, 119400 (2020).
- Baddour, F. G., et al. An Exceptionally mild and scalable solution-phase synthesis of molybdenum carbide nanoparticles for thermocatalytic CO2 hydrogenation. Journal of the American Chemical Society. 142 (2), 1010-1019 (2019).
- Yan, P., et al. Methanol oxidative dehydrogenation and dehydration on carbon nanotubes: active sites and basic reaction kinetics. Sustainable Energy Fuels. 10, 4952-4959 (2020).
- Unocic, R. R., Jungjohann, K., Mehdi, B. L., Browning, N. D., Wang, C. In situ electrochemical scanning/transmission electron microscopy of electrode-electrolyte interfaces. MRS Bulletin. 45, 1-8 (2020).
- LaGrow, A. P., Lloyd, D. C., Gai, P. L., Boyes, E. D. In situ scanning transmission electron microscopy of Ni nanoparticle redispersion via the reduction of hollow NiO. Chemistry of Materials. 30 (1), 197-203 (2017).
- Liu, L., Zakharov, D. N., Arenal, R., Concepcion, P., Stach, E. A., Corma, A. Evolution and stabilization of subnanometric metal species in confined space by in situ TEM. Nature Communications. 9 (1), 574 (2018).
- Wu, Y. A., et al. Visualizing redox dynamics of a single Ag/AgCl heterogeneous nanocatalyst at atomic resolution. ACS Nano. 10 (3), 3738-3746 (2016).
- Li, Y., et al. Complex structural dynamics of nanocatalysts revealed in operando conditions by correlated imaging and spectroscopy probes. Nature Communications. 6 (1), 7583 (2015).
- Hansen, P. L., et al. Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science. 295 (5562), 2053-2055 (2002).
- Creemer, J. F., et al. Atomic-scale electron microscopy at ambient pressure. Progress in Materials Science. 108, 993-998 (2008).
- Was, G. S., Petti, D., Ukai, S., Zinkle, S. Materials for future nuclear energy systems. Journal of Nuclear Materials. 527, 151837 (2019).
- Unocic, K. A., Yamamoto, Y., Pint, B. A. Effect of Al and Cr content on air and steam oxidation of FeCrAl alloys and commercial APMT alloy. Oxidation of Metals. 87 (3-4), 431-441 (2017).
- Zinkle, S. J., et al. Fusion materials science and technology research opportunities now and during the ITER era. Fusion Engineering and Design. 89 (7-8), 1579-1585 (2014).
- Quadakkers, W. J., Olszewski, T., Piron-Abellan, J., Shemet, V., Singheiser, L. Oxidation of metallic materials in simulated CO2/H2O-rich service environments relevant to an oxyfuel plant. Materials Science Forum. 696, 194-199 (2011).
- Gleeson, B. Thermal barrier coatings for aeroengine applications. Journal of Propulsion and Power. 22 (2), 375-383 (2006).
- Unocic, K. A., Allard, L. F., Coffey, D. W., More, K. L., Unocic, R. R. Novel method for precision controlled heating of TEM thin sections to study reaction processes. Microscopy and Microanalysis. 20, 1628-1629 (2014).
- Idrobo, J. C., et al. Temperature measurement by a nanoscale electron probe using energy gain and loss spectroscopy. Physical Review Letters. 120 (9), 095901 (2018).
- Unocic, K. A., Datye, A. K., Bigelow, W. C., Allard, L. F. Water vapor in closed-cell in situ gas reactions: Initial experiments. Microscopy and Microanalysis. 23 (1), 940-941 (2017).
- Allard, L. F., Meyer, H. M., Hensley, D. K., Bigelow, W. C., Unocic, K. A. Model “alloy” specimens for MEMS-based closed-cell gas-reactions. Microscopy and Microanalysis. 23 (1), 908-909 (2017).
- Allard, L. F., et al. The utility of Xe-plasma FIB for preparing aluminum alloy specimens for MEMS-based in situ double-tilt heating experiments. Microscopy and Microanalysis. 25 (2), 1442-1443 (2019).
- Schilling, S., Janssen, A., Zaluzec, N. J., Burke, M. G. Practical aspects of electrochemical corrosion measurements during in situ analytical transmission electron microscopy (TEM) of austenitic stainless steel in aqueous media. Microscopy and Microanalysis. 23 (4), 741-750 (2017).
- Zhong, X. L., Schilling, S., Zaluzec, N. J., Burke, M. G. Sample preparation methodologies for in situ liquid and gaseous cell analytical transmission electron microscopy of electropolished specimens. Microscopy and Microanalysis. 22 (6), 1350-1359 (2016).
- Duchamp, M., Xu, Q., Dunin-Borkowski, R. E. Convenient preparation of high-quality specimens for annealing experiments in the transmission electron microscope. Microscopy and Microanalysis. 20 (6), 1638-1645 (2014).
- Unocic, K. A., Mills, M. J., Daehn, G. S. Effect of gallium focused ion beam milling on preparation of aluminum thin foils. Journal of Microscopy. 240 (3), 227-238 (2010).
- Unocic, R. R., et al. Probing battery chemistry with liquid cell electron energy loss spectroscopy. Chemical Communications. 51 (91), 16377-16380 (2015).

