Dosage-Adjusted Resistance Training in Mice with a Reduced Risk of Muscle Damage
Summary
The present protocol describes a unique technique called dosage-adjusted resistance training (DART), which can be incorporated into precision rehabilitation studies performed in small animals, such as mice.
Abstract
Progressive resistance training (PRT), which involves performing muscle contractions against progressively greater external loads, can increase muscle mass and strength in healthy individuals and in patient populations. There is a need for precision rehabilitation tools to test the safety and effectiveness of PRT to maintain and/or restore muscle mass and strength in preclinical studies on small and large animal models. The PRT methodology and device described in this article can be used to perform dosage-adjusted resistance training (DART). The DART device can be used as a standalone dynamometer to objectively assess the concentric contractile torque generated by the ankle dorsiflexors in mice or can be added to a pre-existing isokinetic dynamometry system. The DART device can be fabricated with a standard 3D printer based on the instructions and open-source 3D print files provided in this work. The article also describes the workflow for a study to compare contraction-induced muscle damage caused by a single bout of DART to muscle damage caused by a comparable bout of isometric contractions (ISOM) in a mouse model of limb-girdle muscular dystrophy type 2B/R2 (BLAJ mice). The data from eight BLAJ mice (four animals for each condition) suggest that less than 10% of the tibialis anterior (TA) muscle was damaged from a single bout of DART or ISOM, with DART being less damaging than ISOM.
Introduction
Exercise confers numerous health benefits on skeletal muscle (reviewed in Vina et al.1). Specifically, progressive resistance training (PRT), which involves performing muscle contractions against progressively greater external loads (e.g., barbells, dumbbells, cable-pulley-weight circuits), is known to help increase muscle mass and strength in both healthy individuals and patient populations (reviewed in previous publications2,3). PRT is based on the overload principle, which states that, when the muscle contracts against progressively greater external loads, it adapts by increasing its physiological cross-sectional area as well as force-producing capacity4. Existing models of PRT in rodents include ladder climbing with resistance applied to the tail, co-contraction of agonist muscles against resistance from antagonists, running with a weighted harness, a squatting exercise elicited by an electrical shock, and resisted wheel-running5,6,7,8,9,10 (reviewed in previous publications11,12). However, there are currently no research tools to perform precisely muscle-targeted, dosage-adjusted PRT in mice that closely resemble the PRT methods and devices used in human clinical research and practice12,13. This limits the ability of investigators to study the safety and effectiveness of precisely dosed PRT in basic and preclinical studies in mice.
To overcome this barrier, a PRT methodology and device are developed in this study based on the cable-pulley-weight circuit designs employed in resistance training equipment in modern gymnasiums14,15,16. This method of PRT is referred to as dosage-adjusted resistance training (DART), and the device is called the DART device. In addition to its functionality as a precision rehabilitation training tool, the DART device can also be used as a standalone instrument to objectively assess the maximum concentric contractile torque that can be generated by the tibialis anterior (TA) muscle in a mouse, similar to how the one-repetition maximum (1RM, the maximum load that can be successfully lifted/moved/pressed/squatted just one time while maintaining good form) is assessed in humans17,18. The DART device can also be coupled with a custom-built or commercial isokinetic dynamometer to measure the peak isometric tetanic force produced by the TA muscle in a mouse (comparable to maximum voluntary contraction [MVC] in humans) and then perform dosage-adjusted PRT with a resistance that is based on the peak tetanic force (e.g., 50% of the peak force).
This article describes the construction of the DART device and explains how it can be coupled with a custom-built dynamometer, which has been described in prior publications19,20,21,22, to assess contractile torque and perform DART. The study also describes how the DART device was used to compare exercise-induced muscle damage caused by a single bout of DART (4 sets of 10 concentrically biased contractions with 50% 1RM) to damage caused by a comparable bout of isometric contractions (4 sets of 10 isometric contractions) in a mouse model of limb-girdle muscular dystrophy type 2B (LGMD2B, or LGMDR2)23,24. The mouse model that was studied lacks a protein called dysferlin, which plays an important role in protecting skeletal muscle against delayed-onset muscle damage following injurious eccentric contractions22,25,26,27,28,29,30. It has also been demonstrated in dysferlin-deficient male mice that concentrically biased forced exercise is not as damaging as eccentrically biased forced exercise and that prior exposure to concentrically biased training offers protection against injury from a subsequent bout of eccentrically biased contractions22. Since the current study was conducted to test the feasibility of the present DART methodology and device in performing dosage-adjusted, concentrically biased resistance training, male dysferlin-deficient mice were chosen for the investigation to compare new data from the DART device with previous data. In future studies, female BLAJ mice will be included to study the effect of sex as a biological variable in relation to the response to DART. Mice that were ~1.5 years old were studied since they already have dystrophic changes in many muscle groups and, therefore, model the pathophysiological state in which muscles might be in patients who already have muscle weakness and wasting and are seeking rehabilitative care to maintain muscle mass and strength26.
Protocol
The experiments described in this article were approved by the Institutional Animal Care and Use Committee (IACUC) at Wayne State University, Detroit, Michigan, USA, in accordance with the Guide for the Care and Use of Laboratory Animals (1996, published by National Academy Press, 2101 Constitution Ave. NW, Washington, DC 20055, USA). B6.A-Dysfprmd/GeneJ mice (a.k.a. BLAJ mice, males, ~1.5 years old) that model LGMD2B/R2 were used for the present study. The mice were obtained from a commercial source (see Table of Materials).
1. Study design
- Choose mouse strain(s) relevant to the research question(s) — e.g., study B6.A-Dysfprmd/GeneJ mice (BLAJ mice) if trying to answer the question as to whether or not concentrically biased DART induces widespread muscle damage in mice that model LGMD2B/R2.
- Assign mice to study groups based on the study design-e.g., randomly assign mice to a dosage-adjusted resistance training (DART) group or to an isometric training group (ISOM), and attempt to balance the groups as best as possible based on matching by litter and/or age (e.g., Table 1).
2. Fabrication of the DART device
- Design the DART device components with suitable computer-aided (CAD) software (Figure 1) following the steps below.
- Design housing for low friction wheel bearing (see Table of Materials, based on pillow block bearing design) with a built-in protractor (for use as a goniometer to measure ankle joint angles).
- Design a tower for wheel bearing housing plus a protractor.
- Design a footplate for positioning the mouse's foot. Design an axle to connect the footplate to the wheel bearing.
- Manufacture the DART device components with a suitable 3D printer (Figure 1).
- Save the designs created with CAD software as stereolithography (.STL extension) files.
NOTE: The .STL files (Supplementary Coding Files 1-4) may be used and modified by giving credit to the corresponding author of this article and citing this article. - Open the .STL files with suitable slicing software (see Table of Materials).
NOTE: Slicing software converts a virtual 3D model into a stack of slices, which can be printed sequentially by a 3D printer to generate a 3D object. - With slicing software, generate G-CODE computer-aided manufacturing (CAM, .GCODE extension) files, which are specific to the 3D printer and filament that will be used.
- Follow the 3D printer manual (see Table of Materials) to print DART device components with .GCODE files.
- Choose an appropriate 3D printer filament, such as polylactic acid (PLA) 1.75 mm 1 kg/spool, Gray (see Table of Materials).
- Save the designs created with CAD software as stereolithography (.STL extension) files.
- Assemble the DART device following the steps below.
- Insert a 608 low-friction wheel bearing (8 mm bore diameter, 22 mm outside diameter, such as one with silicon nitride ceramic balls housed in 420 stainless steel, see Table of Materials) into the wheel bearing housing (Figure 1).
- Insert the axle into the bore of the wheel bearing (Figure 1).
- Paste the footplate onto the axle with glue (see Table of Materials) that is suitable to bond PLA (Figure 1).
- Place the wheel bearing housing above the wheel bearing housing tower and attach the whole assembly to an acrylic base with screw fasteners (Figure 1).
NOTE: There are no specific size requirements for the acrylic base — it just needs to be large enough to accommodate the animal and DART device and small enough to fit on a work surface. The acrylic base used for the present study is about 30 cm wide, 45 cm long, and 0.5 cm thick.
3. Preparation of mice for DART or ISOM
- Place each mouse under general anesthesia with inhaled isoflurane delivered through a suitable anesthesia system (see Table of Materials, 2%-5% for induction; 1%-4% for maintenance; to effect) to reduce stress and pain.
- Induce anesthesia in the induction chamber of the anesthesia system (2%-5% isoflurane).
- Transfer the mouse to a nose cone to maintain anesthesia while performing procedures on the animal (1%-4% isoflurane). Confirm anesthesia effectiveness based on the lack of hindlimb withdrawal to a toe pinch from a pair of tweezers.
- Provide thermal support — e.g., with an isothermal gel heating pad and a heat lamp placed ~1 m above the mouse. Check with a thermometer to ensure that the temperature on and around the acrylic base is maintained at ~38 °C, so the mouse does not overheat.
- Prepare the skin over the left tibialis anterior (TA) muscle of the mouse and over the entire anterior and lateral aspects of the left hindlimb for DART or ISOM.
- Remove the mouse's fur with a hair removal cream (depilatory cream, see Table of Materials). Apply depilatory cream and allow it to work for ~2 min.
- Clean the leg with wipes soaked in distilled water to remove fur and all residual cream from the skin. Depilatory creams can irritate and/or damage the skin if left on the mouse's skin for long periods and, therefore, remove completely.
- After fur removal, disinfect the skin with an approved scrubbing method, such as with a povidone-iodine scrubbing solution and 70% ethanol.
- Apply a protectant (e.g., petrolatum) over the eyes and depilated skin with a clean cotton swab to protect the eyes and depilated skin from drying.
- Place a stabilizing pin through the tibial metaphysis.
- Apply 5% lidocaine cream over the tibia to numb the area.
- Pass a 26 G, half-inch, sterile, hypodermic needle through the widest part of the proximal portion of the tibial bone (i.e., the tibial metaphysis, also known as the tibial head). Once the stabilizing pin is secured, remove the plastic portion of the hypodermic needle by holding the needle with a sterile hemostat and bending the plastic portion until it breaks off.
- Position the mouse for DART or ISOM training.
- Lay the mouse in a supine position. Ensure that the mouse is still securely connected to the nose cone to maintain anesthesia.
- With a pair of sterile-tipped tweezers, feed the tibial pin into a metal alligator clip (see Table of Materials), such that the ends of the tibial pin are held by the alligator clamp. Move the adjustable arm of the alligator clamp to ensure that the mouse's foot is placed on the footplate of the DART device.
- Strap the mouse's foot onto the DART device footplate with adhesive laboratory tape.
- Place the mouse's foot at a 90° angle in relation to the long axis of the mouse's tibial bone. If placed correctly, the footplate will be perpendicular to the acrylic base (i.e., the floor or what is considered the horizontal plane).
- Rest the footplate on the plantarflexion stop created by placing an 18 G, 1.5 in long hypodermic needle through the predrilled holes on the DART device's protractor (Figure 1).
4. DART or ISOM training
- Optimize the electrode placement by placing a bipolar, transcutaneous, neuromuscular electrical stimulation (NMES, see Table of Materials) electrode on the inferolateral aspect of the mouse's knee joint (Figure 1B).
- With single pulses (1 Hz) from a laboratory electrical stimulator (see Table of Materials), stimulate the fibular branch of the sciatic nerve, which provides motor innervation to the ankle dorsiflexor muscles (Figure 1B).
- Since the tibialis anterior (TA) muscle accounts for over 90% of the total contractile force produced by the ankle dorsiflexor muscles31, observe the TA muscle belly and tendon for evidence of electrically elicited twitch contractions.
NOTE: A slight bony prominence that corresponds with the fibula bone might help with electrode placement if the tester can feel it through the electrode. This requires some practice and learning on the part of the tester to get a feel for optimal electrode placement. - Move the plantarflexion stop to the hole on the protractor that corresponds to 20° of plantarflexion from the position at which the foot is orthogonal (90°) to the tibia — this is the position at which maximal contractile torque from the TA muscle is typically observed based on previous reports21. This might have to be customized by the user based on factors specific to the mice that are being studied.
- Visualize twitch torque with a mouse dynamometer by linking the DART device footplate to the dynamometer footplate — e.g., link the DART device footplate to a custom-built robotic ankle dynamometer footplate with a non-elastic silk suture (similar to Figure 1A) and strap the suture to the dynamometer footplate (see Table of Materials).
NOTE: The footplate has holes built into the 3D print design. Placing the suture through the pair of holes that are in the second row from the toe-end of the footplate puts the suture at ~20 mm from the axis of dorsiflexion/plantarflexion (Figure 1A, B). The dynamometer has been described in previous reports19,20,21,22.
- Optimize the voltage output from the NMES stimulator.
- After optimizing the electrode placement, optimize the amplitude of the voltage output from the electrical stimulator — this is necessary to confine NMES to the common fibular nerve and TA muscle and reduce the risk of eliciting co-contractions in the plantarflexors.
NOTE: If co-contractions are elicited, they can be visualized through the torque output from the dynamometer and also be seen in the plantarflexing of the toes.
- After optimizing the electrode placement, optimize the amplitude of the voltage output from the electrical stimulator — this is necessary to confine NMES to the common fibular nerve and TA muscle and reduce the risk of eliciting co-contractions in the plantarflexors.
- Set the NMES stimulator for DART or ISOM training.
NOTE: The following settings might have to be customized by the user based on factors specific to the mice that are being studied and the purpose of the studies.- Set the stimulator to produce repeated pulse trains that are 125 Hz in frequency — this frequency produces maximal fused tetanic contractions without overflow of NMES into other muscle groups in BLAJ mice21. Perform this by adjusting the dials for pulse frequency (125 Hz), train duration (500 ms), and trains per second (1 train/s) and turning on the toggle switch for repeating pulse trains.
- Set stimulator to produce pulse trains that are 500 ms in duration interspersed with 500 ms rest between pulse trains.
- Move the plantarflexion stop to the hole on the protractor that corresponds to 160° to the long axis of the tibia (70° plantarflexion from foot orthogonal to the tibia). This is the position to which the BLAJ mouse's foot can be moved passively without soft tissue resistance21.
- For DART, apply a suitable resistance against which the TA muscle has to work concentrically — e.g., 5 g as shown in Figure 1A, B; see the weight to torque calibration curve in Supplementary File 1.
- Apply resistance by hanging the weight with a non-elastic silk suture that is tied to the DART device footplate (Figure 1A, B).
- Adjust the resistance – i.e., apply ~50% of the one-repetition maximum (1RM) (e.g., 5 g if the mouse can lift a maximum weight of 10 g with a single contraction), which pulls the foot through at least half of the available active range of dorsiflexion.
- Perform suitable DART training in mice assigned to the DART group — e.g., perform a single bout of DART training, which involves four sets of 10 repetitions of concentric contractions with 2 min rest between sets, similar to progressive resistance training programs used in humans32 (see Supplementary Video 1).
- Perform suitable ISOM training in mice assigned to the ISOM group — e.g., perform a single bout of ISOM training, which involves four sets of 10 repetitions of isometric contractions with 2 min rest between sets, similar to DART (see Supplementary Video 2).
- For ISOM training, place the mouse's foot at 160° to the long axis of the tibia (70° plantarflexion from foot orthogonal to the tibia), and maintain this static position by taping the silk suture to the footplate of the robotic dynamometer.
NOTE: Since the suture cannot slide, the DART device footplate cannot move into dorsiflexion, thus constraining the dorsiflexors to contract isometrically.
5. Post-procedural care for mice
- Take precautions to maintain proper hygiene of the exercised hindlimb and reduce needle site pain.
- After the DART or ISOM training, coat the visible portion of the tibial pin with Triple antibiotic ointment (400 U/g of bacitracin, 3.5 mg/g of neomycin, and 5000 U/g of polymixin-B, see Table of Materials) and then withdraw the pin carefully from the medial side of the tibia. Rinse the skin over the lateral thigh and upper leg with povidone-iodine and sterile water. Apply 5% lidocaine cream over the tibia to control needle site pain.
- Allow the mice to recover from anesthesia.
- Remove the mouse from the nose cone and allow it to recover from anesthesia in a recovery cage that is free of bedding. Provide thermal support to the mouse while it recovers from anesthesia, e.g., with an isothermal gel heating pad.
- Return the mouse to its original cage after it completely recovers from anesthesia. Then, return the cage to the animal facility, where study mice are housed until follow-up experiments are performed. Monitor the mice daily.
6. Tissue collection
- Harvest mouse TA muscle in its entirety and snap freeze for cryopreservation following the steps below.
- Based on the research question(s), at a suitable time after training (e.g., 3 days post DART or ISOM), euthanize the mice according to approved protocols.
NOTE: For the present study, mice were euthanized by cervical dislocation under general anesthesia (inhaled isoflurane, 2%-5% to effect). Bilateral thoracotomy ensured death. - Dissect the mouse hindlimbs to remove the exercised TA muscle (left) and unexercised TA muscle (right). Weigh the harvested muscles. Then, dip each muscle in mineral oil for cryoprotection and place the muscle on a clean lab wipe to blot the excess oil21.
- Based on the research question(s), at a suitable time after training (e.g., 3 days post DART or ISOM), euthanize the mice according to approved protocols.
- Place the muscle on a piece of aluminum foil. Hold the edge of the foil with a long hemostat and rapidly immerse the foil and muscle into liquid nitrogen contained in a suitable plastic container to snap freeze the muscle.
- After about 2 min of immersion in liquid nitrogen, transfer the frozen muscle to labeled cryogenic vials. Store vials in a −80 °C freezer until needed for further studies.
7. Histological studies on muscle tissue
- Prepare cryostat sections of TA muscle that are 5 µm in thickness. Collect cryostat sections onto charged microscope slides. Fix the sections with acetone that is kept cold at −30 °C and allow the sections to air dry.
- Stain the muscle tissue sections with hematoxylin followed by eosin (H&E staining, see Table of Materials).
- Immerse the sections for 5 min in hematoxylin (dark blue nuclear stain) in a glass staining jar. Remove excess hematoxylin by rinsing the sections with tap water until no further bluing of water is seen.
- Immerse the sections for 5 min in bluing reagent in a glass jar. Aspirate excess bluing reagent from the sections with a glass suction pipette.
- Immerse the sections for 5 min in eosin (pink cytoplasmic stain) in a glass staining jar. Remove excess eosin by dipping the sections quickly and repeatedly (~10 times) into 95% ethanol in a glass staining jar.
- Allow the sections to air dry and proceed to visualize under a light microscope.
- Prepare high-resolution tiled images of entire TA muscle cross-sections through microscope imaging.
NOTE: The user might have to customize the imaging and image analysis steps that follow based on their microscope and image acquisition and analysis software.- Capture digital images with the 10x objective lens of a light microscope and a digital camera mounted on the microscope.
- Capture about 15-20 images, moving along the cross-section of each muscle in a grid-like manner, such that each new image overlaps ~25% with the previous image.
NOTE: This process helps capture a set of images that can be digitally tiled (also known as image stitching) to create a high-resolution composite image of the entire TA muscle cross-section (Figure 2). - Save digital images in .TIFF format.
- Open digital images with suitable image processing and analysis software (see Table of Materials).
- Tile or stitch individual images into a composite image of the whole TA muscle through the following steps: with all the individual overlapping images of each TA muscle open in software, click on File > Select Automate > Select Photomerge > Select Collage > Select Add Open Files > Click OK.
- When a new tiled/stitched image of the TA muscle is prepared and displayed, save the image in .TIFF format for further analyses.
- Quantify muscle damage by visual analysis in the tiled images of the entire TA muscle with suitable image analysis software.
- In the image analysis software, select the Measure function in the Analyze menu to outline and measure the area of the entire TA muscle cross-section (Figure 2).
- In the image analysis software, select the Measure function in the Analyze menu to outline and measure the areas of each TA muscle that are damaged — i.e., areas that show cytoplasmic disruption of muscle fibers, absent muscle fibers, and inflammatory cell infiltration22 (Figure 2).
- Express the sum of the total area of damage as a percentage of the entire TA muscle cross-sectional area (Figure 2, Table 2).
8. Statistical analyses
- Organize data as shown in Tables 1-3 and perform non-paired T-tests (if tests of normality and homogenous variances are passed)33 or Mann-Whitney Rank Sum tests (if tests of normality and homogenous variances are not passed)21 with suitable software (see Table of Materials).
Representative Results
BLAJ male mice, which were ~1.5 years in age, were studied. BLAJ mice model the human muscle disease, LGMD2B/R2. These mice are particularly susceptible to delayed onset muscle damage from a single bout of eccentric muscle contractions22,29. BLAJ mice were, therefore, chosen for these studies to learn if DART could be performed in a non-injurious manner by precisely adjusting the resistance against which the TA muscle has to work in a concentrically biased manner. If it were found that DART was non-injurious to BLAJ mice, then it would likely be useful as a form of non-injurious resistance training, which could be applied alone or as an adjunct to regenerative medicine, genetic, pharmacological, and other interventions.
The ages and weights of BLAJ mice were closely matched between the DART and ISOM groups (Table 1). On Day 3 (~72 h), after a single bout of training, the exercised TA muscle had low levels of damage in both the DART and ISOM groups (<10% damaged area) — this is in contrast to past studies21,22 of the response of BLAJ mice to eccentric muscle contractions, where ~40% damaged fibers have been reported at Day 3 (Figure 2, Table 2). When the area of muscle damage was compared between exercised TA muscles from the DART and ISOM groups, it was found that the DART group had lower levels of muscle damage than the ISOM group (Figure 2, Table 2). The maximum tetanic torque recorded on Day 0 (baseline) and Day 3 was not statistically different between the DART and ISOM groups (Table 3).
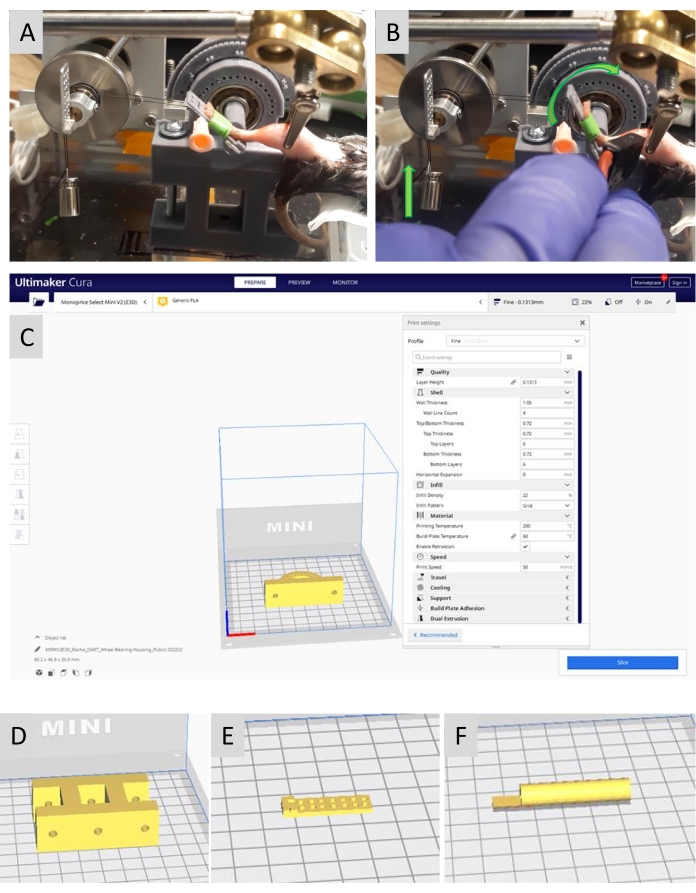
Figure 1: Fabricating the DART device and applying it in a training study. (A,B) The DART device is based on a cable-pulley-weight circuit design, which is common to resistance training equipment that is designed for humans. (A) The DART device with an animal during a DART training session. (B) The footplate moving into dorsiflexion during a concentric contraction of the TA muscle (curved green arrow, right). The concentric contraction causes the 5 g resistance to move vertically against gravity (vertical green arrow, left). Muscle contractions were elicited with electrical stimulation applied through a transcutaneous bipolar electrode. (C) Various components of the DART device were designed with stereolithography software to generate .STL files, which could be opened with slicing software. With slicing software, G-CODE files were generated specific to the 3D printer and filament used. The 3D printed components of the DART device included (C) housing for a 608 low friction wheel bearing, (D) a tower for the wheel bearing housing, (E) a footplate, and (F) an axle to connect the footplate to the wheel bearing. The 3D printed components were combined and mounted on an acrylic base with glue and screw fasteners as described in the text and shown in (A). Please click here to view a larger version of this figure.
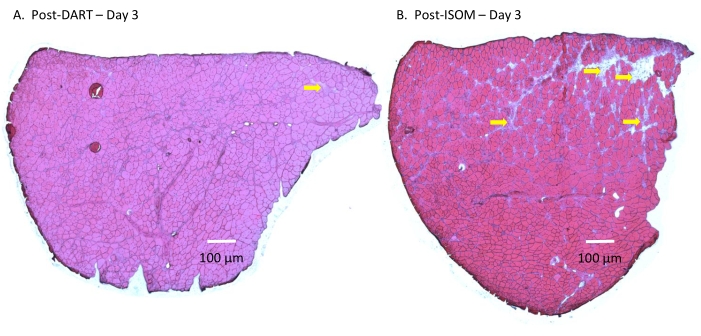
Figure 2: Histological study. Histological changes in the TA muscle at Day 3 (A) post-DART or (B) post-ISOM. Cryosections, which were 5 µm in thickness, were stained with hematoxylin and eosin. Multiple overlapping digital images were captured and merged together with imaging software to generate high-resolution tiled images of the entire TA muscle cross-section. The qualitative histological data indicated that the extent of muscle damage was low in both DART and ISOM groups, but muscle damage was slightly more obvious in the ISOM group. The yellow arrows point to some of the damaged regions in TA muscle cross-sections. Please click here to view a larger version of this figure.
Table 1: Ages and body weights of mice. The BLAJ mice that were studied were closely matched in age and body weight with no significant difference between the DART and ISOM groups. Please click here to download this Table.
Table 2: Quantitative analysis of TA muscle damage. The extent of muscle damage was expressed as a percentage of the total area of the TA muscle cross-section and analyzed by a T-test. Both DART and ISOM training resulted in a low level of muscle damage at Day 3 when compared to past studies involving a similar bout of eccentric contractions in BLAJ mice. Though the magnitude of muscle damage was small in both the DART and ISOM groups, the extent of damage was statistically lower in the DART group. Please click here to download this Table.
Table 3: Contractile torque data. Contractile torque produced by the dorsiflexor muscles was studied with a robotic dynamometer connected to the DART device. There was no significant difference between the DART and ISOM groups in maximum baseline tetanic torque measured on the day of exercise (A, Day 0) or at 3 days post exercise (B, Day 3). Despite the lack of histological evidence of widespread muscle damage, a single bout of DART and ISOM was associated with a contractile torque deficit (~40%) on Day 3. Please click here to download this Table.
Supplementary Video 1: DART training in mice. Please click here to download this Video.
Supplementary Video 2: ISOM training in mice. Please click here to download this Video.
Supplementary File 1: Weight to torque calibration data, curve, and setup. Please click here to download this File.
Supplementary Coding Files 1-4: Designs for the DART device components. Please click here to download this File.
Discussion
This article presents step-by-step instructions on how to construct a device to perform a type of precision rehabilitation training called dosage-adjusted resistance training (DART). The work also describes the application of the DART device and methodology in a training study to compare muscle damage 3 days after a single bout of DART (DART group) with damage 3 days after a comparable bout of isometric training (ISOM group).
The critical steps in the protocol are the proper construction of the DART device34,35, the precise steps involved in performing DART or ISOM training, the proper harvesting and cryopreservation of muscle tissue, the proper sectioning of muscle tissue with a cryostat, and the proper staining of muscle cross-sections with hematoxylin and eosin22,36. Specifically, to construct the DART device, the parts must be fabricated with the exact dimensions and optimal material properties. If the dimensions are inaccurate for the wheel bearing housing, the 608-type wheel bearing will not fit snugly within the wheel bearing housing. If the dimensions of the mouse footplate and axle are not accurate, it might adversely impact the ability of the wheel bearing to move along with the mouse's foot. If the DART device parts are fabricated with an unsuitable material and/or 3D printer settings, the DART device parts might lack sufficient mechanical strength, which might lead to bending and/or breaking of various components34.
Modifications of this protocol might be needed based on the specific research questions that investigators wish to answer. The current protocol is specific to designing and implementing the DART device in a study that attempted to answer the question as to whether or not a single bout of DART causes extensive damage to the TA muscle in dysferlin-deficient mice, as we reported earlier with a similar bout of eccentric contractions22. Since others have suggested that exercise consisting of isometric contractions might be non-injurious and, therefore, suitable for humans with certain muscle diseases, we compared the extent of muscle damage caused by DART to a comparable bout of isometric contractions (ISOM)37,38. In this study, we found that both DART and ISOM induce minimal muscle damage, with DART showing slightly but significantly lower levels of damage than ISOM.
In relation to troubleshooting, the most challenging aspect of the protocol is precisely stimulating the fibular branch of the sciatic nerve, which gives motor innervation to the TA muscle. This technique is particularly challenging because the tester holds a transcutaneous electrode and places it manually on a precise spot that is inferior and lateral to the mouse's knee joint20,39. The tester must practice and learn how to locate this spot on the mouse's hindlimb by feeling for a slight bony prominence corresponding to the head of the mouse's fibular bone40. In order to confirm that optimal electrical stimulation of the fibular branch of the sciatic nerve is being achieved, such that maximal contractions from the TA muscle are achieved, it is best that a reliable dynamometery system is used20,21,22,41. Furthermore, transcutaneous or subcutaneous electrodes stabilized by a clamp may also be considered for reliable and reproducible placement of electrodes to minimize user-induced variability and errors20,41,42,43.
The main limitation of the protocol is that it is specifically designed to study the effect of DART on the TA muscle in mice. With methods that have been developed to perform dynamometric assessments and forced exercise on the quadriceps femoris muscle group in rodents, the DART device can easily be adapted for the quadriceps femoris muscle group42,43. Applying the DART device to other muscle groups might be more challenging; however, the cable-pulley-weight circuit design, which has been used in the DART device, can be incorporated into devices that are suitable for other muscle groups. Another limitation is that the protocol is performed under general anesthesia, making exercise forced and not voluntary; this is different from most resistance training paradigms developed for humans12,21.
The significance of the DART device and methodology with respect to existing or alternative methods is that the dosage for resistance training can be precisely adjusted and the exercise can be precisely targeted to a particular muscle group12. Precision rehabilitation is a new strategic priority for the United States National Institutes of Health, and, since DART makes it possible to perform precision resistance training in mice, DART lends itself well to basic and preclinical studies on precision physical rehabilitation44,45.
The importance and potential application of the current method of performing dosage-adjusted resistance training are that it makes it possible to perform resistance training studies in mice in ways comparable to human testing and training protocols used in clinical rehabilitation research and practice. For example, just as the one-repetition maximum (1RM, the maximum load that can be successfully lifted/moved/pressed/squatted just one time while maintaining good form) is used for humans to adjust the magnitude of resistance for training bouts17,18, the maximum load that the TA muscle can successfully lift can be used to set the resistance for training in mice with the DART device. In addition to adjusting the resistance based on an animal's capacity, the additional advantage is that the contractions are concentrically biased, which helps reduce contraction-induced muscle injury22. The representative results suggest that one bout of DART is even less injurious than a comparable bout of isometric contractions (ISOM group). The non-injurious nature of DART makes it appropriate for training studies where injurious contractions are best avoided — e.g., training studies in mice that model muscular dystrophies and training studies designed to gradually reload muscle following experimental surgical procedures on muscles and/or tendons22,46,47.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was funded by grants from the Jain Foundation Inc., R03HD091648 from NICHD, a Pilot Grant from AR3T under NIH P2CHD086843, a FRAP Award from EACPHS at Wayne State University, a Faculty Startup Package from Wayne State University, and a subcontract from 1R01AR079884-01 (Peter L. Jones PI) to JAR. This study was also funded by an American Physical Therapy Association – Michigan (APTA-MI) research grant to JMB, MEP, and JAR. The authors acknowledge Dr. Renuka Roche (Associate Professor, Eastern Michigan University, MI) for critically reading the manuscript and providing feedback. The authors acknowledge Mr. Anselm D. Motha for advice on 3D printing. The authors thank the patients with dysferlinopathies who have shared their stories on the Jain Foundation website at https://www.jain-foundation.org/patient-physician-resources/patient-stories, particularly their experiences with exercise.
Materials
| AnMiao Star 608 Ceramic Ball Bearing | Anmiao Star (N/A) | AMS127 | High precision, low friction wheel bearing. If make and model is not commercially available, an alternative version of a 608 low-friction wheel bearing, 8 mm bore diameter, 22 mm outside diameter, with silicon nitride ceramic balls in 420 stainless steel housing should suffice. Excess friction in the wheel bearing will adversely impact performance of the DART device and will increase overall resistance to muscle contractions. |
| Axio Scope.A1 microscope | Carl Zeiss (Peabody, MA) | Product #Axio Scope.A1 | Light and fluorescence microscope |
| B6.A-Dysfprmd/GeneJ (a.k.a. BLAJ mice) | The Jackson Laboratory (Bar Harbor, ME). Special colony maintained by The Jain Foundation Inc. for collaborators who study dysferlin. | Stock #012767 | Dysferlin deficient mice that model human limb girdle muscular dystrophy type 2B/R2. |
| Bipolar, transcutaneous, neuromuscular electrical stimulation (NMES) electrode | Harvard Apparatus, Holliston, MA | BS4 50–6824 | Electrode for NMES. If this electrode is not commercially available, please contact corresponding author for alternatives. |
| Coplin Staining Dish | ThermoFisher (Waltham, MA) | Catalog No. S17495 | Staining dish/jar for hematoxylin and eosin (H&E) staining of sections |
| Cura 4.4.1. Software | Ultimaker, Utrecht, Netherlands | Ultimaker Cura 4.4.1. | Slicing software to convert stereolithography files into G-CODE files |
| Deltaphase isothermal gel heating pad | Braintree Scientific (Braintree, MA) | Item #39DP | Heating pad to provide thermal support to animals while under anesthesia |
| Eosin Y | Millipore Sigma (Burlington, MA) | HT110132-1L | Pink cytoplasmic stain |
| Gorilla Super Glue | The Gorilla Glue Company (Cincinnati, OH) | Gorilla Super Glue Micro Precise | Cyanoacrylate adhesive to bond PLA components |
| Hematoxylin solution, Gill No.3 | Millipore Sigma (Burlington, MA) | GHS332-1L | Dark blue stain for nuclei |
| HM525NX cryostat | ThermoFisher (Waltham, MA) | Catalog #HM525NX | Cryostat to make frozen sections of muscle |
| Lab Wipes. Kimberly-Clark Professional Kimtech Science Kimwipes Delicate Task Wipers, 1-Ply | ThermoFisher (Waltham, MA) | Catalog No. 06-666. Manufacturer #34120 | Laboratory wipes to blot mineral oil from muscle tissue before snap freezing and for other purposes. |
| Labview 2014 | National Instruments, Austin, Texas, USA | Labview 2014 | Software for custom-written programs/routines that operate the dynamometer and trigger the NMES stimulator. |
| Liquid nitrogen HDPE Dewar Flasks | ThermoFisher (Waltham, MA) | S34074B. Thermo Scientific 41502000/EMD | Flask to hold liquid nitrogen for snap freezing muscle or other tissue |
| Magic depilatory cream | Softsheen Carson (New York, NY) | N/A | Razorless hair removal cream |
| Metal alligator clip | JINSHANGTOPK (web-based business) | 24Pcs 51mm Metal Alligator Clip Spring Clamps | Spring clamp to hold tibial pin |
| Micrscope slides | Globe Scientific (Mahwah, NJ) | 1354W. Diamond White Glass Slides | Charged microscope slides |
| Mineral Oil | ThermoFisher (Waltham, MA) | BP26291 | Mineral oil to cryoprotect muscle tissue before snap freezing |
| Monoprice Premium 3D Printer Filament PLA | Monoprice (Rancho Cucamonga, CA) | #11778 | Premium 3D Printer Filament PLA 1.75mm 1 kg/spool, Gray. This is the material used to 3D print device components. |
| Monoprice Select Mini V2 3D printer | Monoprice (Rancho Cucamonga, CA) | Mini V2 3D | 3D printer for computer-aided fabrication of device components. |
| NIH Image software | National Instritues of Health (NIH, Bethesda, MD) | NIH Image for Windows | Image processing and analysis software used to quantify area of muscle damage. NIH Image is also known as Image J. |
| Photoshop CS4 | Adobe (San Jose, CA) | Creative Suite (CS4). 64 bit version for Windows | Image processing and analysis software used to generate tiled/stiched images of entire muscle cross-section from images of indvidual overlapping fields |
| PSIU6 stimulation isolation unit | Grass Instruments (West Warwick, RI) | PSIU6 isolation unit | Isolation unit for NMES. Stimulators, such as Model 4100 from A-M come with a built in stimulation isoloation unit |
| Roboz 4-0 silk black braided suture material | Roboz Surgical (Gaithersburg, MD) | Roboz Surgical SUT152 | Suture material to connect DART device footplate to dynamometer footplate or resistance for resistance training |
| S48 square pulse stimulator | Grass Instruments (West Warwick, RI) | S48 Stimulator | Laboratory electrical stimulator for NMES . If this stimulator is not commercially available, Model 4100 Isolated High Power Stimulator from A-M systems could be an alternative. Please contact co-author Jones for more information. |
| Scott’s bluing reagent | Ricca Chemical Company (Arlington, TX) | 6697-32 | Bluing solution that intensifies hematoxylin nuclear staining |
| SigmaStat version 3.5 | Systat Software (San Jose, CA) | SigmaStat version 3.5 | Statistical software package for statistical analyses |
| Tabletop isoflurane vaporizer | VetEquip (Livermore, CA) | Item #901801 | Inhaled tabletop anesthesia system |
| Triple antibiotic first aid ointment | Global Health Products (wed-based business) | Globe Triple Antibiotic First Aid Ointment, 1 oz (2-Pack) First Aid Antibiotic Ointment | Antibiotic ointment applied on tibial pin as part of post-procedural care |
References
- Vina, J., Sanchis-Gomar, F., Martinez-Bello, V., Gomez-Cabrera, M. C. Exercise acts as a drug; The pharmacological benefits of exercise. British Journal of Pharmacology. 167 (1), 1-12 (2012).
- Murton, A. J., Greenhaff, P. L. Resistance exercise and the mechanisms of muscle mass regulation in humans: Acute effects on muscle protein turnover and the gaps in our understanding of chronic resistance exercise training adaptation. The International Journal of Biochemistry & Cell Biology. 45 (10), 2209-2214 (2013).
- Pepin, M. E., Roche, J. A., Malek, M. H., Chandler, T. J., Brown, L. E. Strength Training for Special Populations. Conditioning for Strength and Human Performance. , 547-570 (2019).
- Helland, C., et al. Training strategies to improve muscle power: Is Olympic-style weightlifting relevant. Medicine and Science in Sports and Exercise. 49 (4), 736-745 (2017).
- Souza, M. K., et al. l-Arginine supplementation blunts resistance exercise improvement in rats with chronic kidney disease. Life Sciences. 232, 116604 (2019).
- Schmoll, M., et al. SpillOver stimulation: A novel hypertrophy model using co-contraction of the plantar-flexors to load the tibial anterior muscle in rats. PloS One. 13 (11), 0207886 (2018).
- Adams, G. R., Haddad, F., Bodell, P. W., Tran, P. D., Baldwin, K. M. Combined isometric, concentric, and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. Journal of Applied Physiology. 103 (5), 1644-1654 (2007).
- Guedes, J. M., et al. Muscular resistance, hypertrophy and strength training equally reduce adiposity, inflammation and insulin resistance in mice with diet-induced obesity. Einstein. 18, (2019).
- Zhu, W. G., et al. Weight pulling: A novel mouse model of human progressive resistance exercise. Cells. 10 (9), 2459 (2021).
- Call, J. A., McKeehen, J. N., Novotny, S. A., Lowe, D. A. Progressive resistance voluntary wheel running in the mdx mouse. Muscle & Nerve. 42 (6), 871-880 (2010).
- Strickland, J. C., Smith, M. A. Animal models of resistance exercise and their application to neuroscience research. Journal of Neuroscience Methods. 273, 191-200 (2016).
- Greising, S. M., Basten, A. M., Schifino, A. G., Call, J. A., Greising, S. M., Call, J. A. Considerations for Small Animal Physical Rehabilitation. Regenerative Rehabilitation: From Basic Science to the Clinic. , 39-59 (2022).
- Roche, J. A., Greising, S. M., Call, J. A. Regenerative Rehabilitation for Nonlethal Muscular Dystrophies. Regenerative Rehabilitation: From Basic Science to the Clinic. , 61-84 (2022).
- Schott, N., Johnen, B., Holfelder, B. Effects of free weights and machine training on muscular strength in high-functioning older adults. Experimental Gerontology. 122, 15-24 (2019).
- . Dr. Gustav Zander’s Victorian-Era Exercise Machines Made the Bowflex Look Like Child’s Play Available from: https://www.smithsonianmag.com/smithsonian-institution/gustav-zander-victorian-era-exercise-machines-bowflex-180957758/ (2016)
- Hansson, N., Ottosson, A. Nobel prize for physical therapy? Rise, fall, and revival of medico-mechanical institutes. Physical Therapy. 95 (8), 1184-1194 (2015).
- ACSM. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Medicine and Science in Sports and Exercise. 41 (3), 687-708 (2009).
- Suchomel, T. J., Nimphius, S., Bellon, C. R., Hornsby, W. G., Stone, M. H. Training for muscular strength: Methods for monitoring and adjusting training intensity. Sports Medicine. 51 (10), 2051-2066 (2021).
- Bloch, R. J., et al. Small-Animal Unit for Muscle Injury, Muscle Testing and Muscle Training in Vivo. US Patent. , (2012).
- Lovering, R. M., Roche, J. A., Goodall, M. H., Clark, B. B., McMillan, A. An in vivo rodent model of contraction-induced injury and non-invasive monitoring of recovery. Journal of Visualized Experiments. (51), e2782 (2011).
- Begam, M., et al. Diltiazem improves contractile properties of skeletal muscle in dysferlin-deficient BLAJ mice, but does not reduce contraction-induced muscle damage. Physiological Reports. 6 (11), 13727 (2018).
- Begam, M., et al. The effects of concentric and eccentric training in murine models of dysferlin-associated muscular dystrophy. Muscle and Nerve. 62 (3), 393-403 (2020).
- Straub, V., Murphy, A., Udd, B. 229th ENMC international workshop: Limb girdle muscular dystrophies – Nomenclature and reformed classification Naarden, the Netherlands. Neuromuscular Disorders. 28 (8), 702-710 (2018).
- . DYSFERLIN Available from: https://www.omim.org/entry/603009 (2021)
- Millay, D. P., et al. Genetic manipulation of dysferlin expression in skeletal muscle: Novel insights into muscular dystrophy. American Journal of Pathology. 175 (5), 1817-1823 (2009).
- Nagy, N., et al. Hip region muscular dystrophy and emergence of motor deficits in dysferlin-deficient Bla/J mice. Physiological Reports. 5 (6), 13173 (2017).
- Roche, J. A., Lovering, R. M., Bloch, R. J. Impaired recovery of dysferlin-null skeletal muscle after contraction-induced injury in vivo. Neuroreport. 19 (16), 1579-1584 (2008).
- Roche, J. A., et al. Extensive mononuclear infiltration and myogenesis characterize recovery of dysferlin-null skeletal muscle from contraction-induced injuries. American Journal of Physiology: Cell Physiology. 298 (2), 298-312 (2010).
- Roche, J. A., Ru, L. W., Bloch, R. J. Distinct effects of contraction-induced injury in vivo on four different murine models of dysferlinopathy. Journal of Biomedicine and Biotechnology. 2012, 134031 (2012).
- Roche, J. A., et al. Myofiber damage precedes macrophage infiltration after in vivo injury in dysferlin-deficient A/J mouse skeletal muscle. American Journal of Pathology. 185 (6), 1686-1698 (2015).
- Ingalls, C. P., Warren, G. L., Zhang, J. Z., Hamilton, S. L., Armstrong, R. B. Dihydropyridine and ryanodine receptor binding after eccentric contractions in mouse skeletal muscle. Journal of Applied Physiology. 96 (5), 1619-1625 (2004).
- Dutton, M. . Orthopaedics for the Physical Therapist Assistant. , 238 (2011).
- Begam, M., Abro, V. M., Mueller, A. L., Roche, J. A. Sodium 4-phenylbutyrate reduces myofiber damage in a mouse model of Duchenne muscular dystrophy. Applied Physiology, Nutrition, and Metabolism. Physiologie Appliquée, Nutrition et Métabolisme. 41 (10), 1108-1111 (2016).
- Tully, J. J., Meloni, G. N. A scientist’s guide to buying a 3D printer: How to choose the right printer for your laboratory. Analytical Chemistry. 92 (22), 14853-14860 (2020).
- Schwiening, C. 3D printing primer for physiologists. Physiology News. (101), (2015).
- Begam, M., Roche, J. A. Damaged muscle fibers might masquerade as hybrid fibers – A cautionary note on immunophenotyping mouse muscle with mouse monoclonal antibodies. European Journal of Histochemistry. 62 (3), 2896 (2018).
- Lott, D. J., et al. Safety, feasibility, and efficacy of strengthening exercise in Duchenne muscular dystrophy. Muscle & Nerve. 63 (3), 320-326 (2021).
- Lindsay, A., Larson, A. A., Verma, M., Ervasti, J. M., Lowe, D. A. Isometric resistance training increases strength and alters histopathology of dystrophin-deficient mouse skeletal muscle. Journal of Applied Physiology. 126 (2), 363-375 (2019).
- Dalkin, W., Taetzsch, T., Valdez, G. The fibular nerve Injury method: A reliable assay to identify and test factors that repair neuromuscular junctions. Journal of Visualized Experiments. (114), e54186 (2016).
- Amend, S. R., Valkenburg, K. C., Pienta, K. J. Murine hind limb long bone dissection and bone marrow isolation. Journal of Visualized Experiments. (110), e53936 (2016).
- Gerlinger-Romero, F., et al. Non-invasive assessment of dorsiflexor muscle function in mice. Journal of Visualized Experiments. (143), e58696 (2019).
- Brightwell, C. R., et al. In vivo measurement of knee extensor muscle function in mice. Journal of Visualized Experiments. (169), e62211 (2021).
- Pratt, S. J. P., Lawlor, M. W., Shah, S. B., Lovering, R. M. An in vivo rodent model of contraction-induced injury in the quadriceps muscle. Injury. 43 (6), 788-793 (2012).
- Shields, R. K. Precision rehabilitation: How lifelong healthy behaviors modulate biology, determine health, and affect populations. Physical Therapy. 102 (1), 248 (2022).
- . Medical Rehabilitation Research Resource Network (MR3N). Precision Rehabilitation – Inaugural Scientific Retreat Available from: https://ncmrr.org/education-training/archived-presentations/precision-rehab-archive (2021)
- Roche, J. A., et al. Minimally invasive muscle embedding generates donor-cell-derived muscle fibers that express desmin and dystrophin. Military Medicine. 185, 423-429 (2020).
- Roche, J. A., et al. Minimally invasive muscle embedding (MIME), facilitates the development of functional muscle fibers of human cadaveric origin, in host mice. The FASEB Journal. 33, 602 (2019).

