Real-Time Dynamic Collection of Hippocampal Extracellular Fluid from Conscious Rats Using a Microdialysis System
Summary
The protocol here provides a detailed real-time dynamic sampling of extracellular fluid from the hippocampus of awake rats using a microdialysis system.
Abstract
A variety of central nervous system (CNS) diseases are associated with changes in the composition of hippocampal extracellular fluid (HECF). However, difficulty in obtaining HECF in real time from conscious rats has long restricted the evaluation of CNS disease progression and the effectiveness of ethnomedicine therapy. Encouragingly, a brain microdialysis technique can be used for continuous sampling with the advantages of dynamic observation, quantitative analysis, and a small sampling size. This allows the monitoring of changes in the extracellular fluid content for compounds from traditional herbs and their metabolites in the brain of living animals. The aim of this study was thus to accurately implant a cerebrospinal fluid microdialysis probe into the hippocampal region of Sprague Dawley (SD) rats with a three-dimensional brain stereotaxic apparatus, cutting off molecular weights greater than 20 kDa. The high-quality HECF was then obtained from conscious rats using a microdialysis sampling control system with an adjustable sampling rate from 2.87 nL/min – 2.98 mL/min. In conclusion, our protocol provides an efficient, rapid, and dynamic method to obtain HECF in awake rats with the help of microdialysis technology, which provides us with unlimited possibilities to further explore the pathogenesis of CNS-related diseases and evaluate drug efficacy.
Introduction
Central nervous system (CNS) diseases with high morbidities, such as neurodegenerative disease, traumatic brain injury, high-altitude hypoxia-induced brain injury, and ischemic stroke, are crucial causes of the growing mortality worldwide1,2,3. Real-time monitoring of cytokines and protein changes in specific brain regions contributes to the diagnostic accuracy of CNS diseases and brain pharmacokinetic studies after medication. Traditional scientific research uses brain tissue homogenate or a manual collection of animal interstitial brain fluid for the detection of specific substances and for pharmacokinetic studies. However, this has some shortcomings, such as a limited sample size, the inability to dynamically observe the changes of indicators, and uneven sampling quality4,5,6. Cerebrospinal fluid, an interstitial fluid, protects the brain and spinal cord from mechanical damage. Its composition is different from that of the serum due to the existence of the blood-brain barrier (BBB)7. Direct analysis of cerebrospinal fluid samples is more conducive to disclosing the mechanism of CNS lesions and drug discovery. Inevitably, the cerebrospinal fluid samples, manually obtained directly from the cisterna magna and cerebral ventricles through a syringe, have disadvantages of blood contamination, a random chance of sample collection, uncertainty of quantity, and almost no multiple retrieval possibility8,9. More notably, conventional interstitial brain fluid sampling methods cannot obtain samples from damaged brain regions, which hinders the exploration of the pathogenesis of CNS diseases in specific brain regions and the efficacy evaluation of targeted ethnomedicine therapies9,10.
Brain microdialysis is a technique for sampling interstitial brain fluid in awake animals11. The microdialysis system imitates vascular permeability with the help of a probe implanted in the brain. The microdialysis probe is armed with a semi-permeable membrane and is implanted in specific brain regions. After perfusion with isotonic artificial cerebrospinal fluid (ACSF), the dialyzed interstitial brain fluid can be favorably collected with the benefits of small sample sizes, continuous sampling, and dynamic observation12,13. In terms of location, brain microdialysis probes can be selectively implanted into brain structures or cranial cisterns of interest14. An observation of abnormal levels of an endogenous substance in the hippocampal extracellular fluid (HECF) suggests the occurrences of CNS diseases or the pathogenesis of disease. Several studies have shown that the biomarkers for CNS diseases, such as D-amino acids in schizophrenia, β-amyloid and tau proteins in Alzheimer's disease, neurofilament light chains in traumatic brain injury, and ubiquitin carboxy-terminal hydrolase L1s in hypoxic ischemia encephalopathy, can be analyzed in cerebrospinal fluid15,16,17. A chemical analysis method based on the brain microdialysis sampling technique can be used to monitor dynamic changes of exogenous compounds such as active ingredients of ethnomedicine, which diffuse and distribute in specific brain regions14.
This article presents the specific process of dynamic HECF acquisition in awake rats and measures its osmotic pressure to ensure sample quality.
Protocol
The experimental protocol was conducted in accordance with the requirements of the Use of Laboratory Animals and Institutional Animal Care and Use Committee at Chengdu University of Traditional Chinese Medicine (Record number: 2021-11). Male Sprague Dawley (SD) rats (280 ± 20 g, 6-8 weeks old) were used for the present study.
1. Brain microdialysis probe implantation surgery
- Use 3% and 1.5% isoflurane for the induction and maintenance of rat anesthesia, respectively, using an animal anesthesia system in an air-oxygen mixture at 0.6 L/min. Make sure that the rats are deeply anesthetized without both a pain reflex and a corneal reflex. Use veterinary ointment on the eyes to prevent dryness while under anesthesia.
- Remove the fur on the anesthetized rat's skull with an electric shaver in the preparation area. Then, fix the anesthetized rat on a stereotaxic brain locator. Disinfect the surgical site before the operation by applying povidone-iodine and ethanol 3x to the region of surgery with a sterile cotton ball. Apply bupivacaine topically for local analgesia. NOTE: The entire process of microdialysis-based HECF sample acquisition is illustrated in Figure 1.
- Make a 1.5 cm craniofacial incision down the middle with surgical scissors and remove the periosteum using surgical scissors and ophthalmic forceps.
- Consider the bregma as the basal position and pierce the endocranium to drill a 2 mm aperture at the anteroposterior (AP) position (-2 mm), the mediolateral (ML) position (-3.5 mm), and the dorsoventral (DV) position (-3.5 mm) (the hippocampal CA1 region) using a cranial drill.
- Fix the catheter stylet on the gripper of a stereotaxic brain locator and adjust the position of the microdialysis casing at the AP (-2 mm), ML (-3.5 mm), and DV (0 mm) positions. Adjust the DV value of the brain stereo locator, and implant the microdialysis casing into the CA1 region at a depth of 3.5 mm.
NOTE: Maintain the temperature of the animal at 37 °C during the operation using an animal temperature maintainer. - Drill three more apertures with a 2 mm diameter such that the three apertures form a triangle in which the probe aperture is centrally located. Implant screws into the apertures at a depth of 1 mm.
- Fix the probe catheter with dental cement and use a 4-0 surgical suture to close the skin. See Figure 2 for the placement of the probe.
- Place the rat in cages for 7 days to recover. Locally infiltrate bupivacaine (1.5 mg/kg) once daily post-surgery. Provide food and water ad libitum. Use sodium hyaluronate eye drops 3x a day to prevent dryness after the operation.
NOTE: Perform all the procedures in a sterile surgical room. Do not leave the animal unattended until it has regained sufficient consciousness to maintain sternal recumbency under a 37 °C condition. Do not return the animal that has undergone surgery to the company of other animals until fully recovered.
2. Microdialysis system connection and probe check
- Connect the microdialysis pump, microsyringe, awake activity device, and cryogenic sample collector according to the manufacturer's instructions. Install the microsyringe with ACSF on the microdialysis pump and set the microdialysis pump to a rate of 1 µL/min to discharge the air in the pipeline.
- Connect the pipeline and brain microdialysis probe (membrane: PAES; membrane length: 4 mm; membrane OD: 0.5 mm; cut-off: 20 kDa; shaft length: 14 mm). Operate the microdialysis pump at a rate of 1 µL/min to inject ACSF into the probe until the surface of the probe is slightly moist. Immerse the probe in heparin sodium injection solution for subsequent use.
NOTE: If a big stream of the ACSF falls from the semipermeable membrane of the probe, as seen with the naked eye under gravity, replace the probe with a new one.
3. Collection of HECF from the awake rat
- Insert the brain microdialysis probe into the probe catheter and place the rat in a chamber (height: 360 mm; diameter: 400 mm) with padding to make sure the rats are free to move around.
- Connect the pipeline, microsyringe pump, and brain microdialysis probe. Harness the rat via the hole at the top of the rat restraint device and the stainless-steel swivels.
- Turn on the multi-channel swivel controller to avoid intertwining the microdialysis pipelines during the free movement of the rat. Turn on the microsyringe pump and pump the ACSF at a rate of 1 µL/min. Collect HECF periodically after a 60 min equilibration of the microdialysis HECF collection system.
- Ensure that the flow rate of the HECF samples in the refrigerated fraction collector is consistent with ACSF infusion. Collect 20 µL of HECF and automatically change to the next sampling tube. Take care to check whether the probe membrane is damaged when inserting the probe.
4. Measurement of the osmotic pressure for the HECF
- Turn on the osmometer and log in to the detection system. Click on the Cal button on the touch screen and click on the Res button on the page to clear the previous calibration memory.
- Install a 1.5 mL tube containing 100 µL of pure water without bubbles on the measuring head. Pull the measuring head to the bottom of the cold hydrazine container.
- Enter the sample number 0 on the touch screen and confirm to test. Quickly dip the diode needle into the sample tube, and then quickly pull it out to induce crystallization of the sample at a temperature of -6.2 °C.
- Wait for the screen to display: Push measure head up and click on Cal and Cal 0 in turn to calibrate. Perform measurements with a 300 mOsm calibration solution and measure the osmotic pressure of the HECF samples as described above.
NOTE: Wipe the measuring head with a soft paper towel after calibration or measurement. The HECF samples with no bubbles should be mixed well.
5. Maintenance of the microdialysis system and devices after sampling
- Take out the brain microdialysis probe from the probe catheter after sampling termination. Immerse the probe in deionized water and lavage with deionized water for 12 h to remove stranded salt depositions from the pipeline and the probe.
- Remove the probe to place into a 0.05% trypsin solution at 4 °C. Dry the pipelines in an air-dry oven at 25 °C and store them at room temperature.
NOTE: The microdialysis probes are expensive and this step can increase the reusability of the probes. Proteins that adhere to the probe surface can be digested by trypsin solution to prevent the probe membrane from being blocked by proteins, and trypsin had no effect on the probe material.
6. Animal treatment after sampling
- After sampling, painlessly euthanize the rats by making them inhale 1.5% isoflurane, followed by an overdose of 5% isoflurane in accordance with animal ethics.
Representative Results
Following the above experimental protocol and the sampling parameters set in Table 1, water-like, colorless, and transparent rat HECF was obtained at the set sampling rate (Figure 1K). The osmotic pressure of the obtained rat HECF was 290-310 mOsm/L, which can indirectly ensure the quality of samples18,19.

Figure 1: Rat HECF collected using the microdialysis sampling equipment. (A,B) The animal anesthesia system and digital-display stereotaxic apparatus were used to anesthetize and immobilize rats. (C) Sampling collection tube for microdialysis system. (D) The cranial anatomical structure of the rat showed the bregma and lambdoidal suture clearly. (E) The catheter stylet and brain microdialysis probe, exhibiting the dialysis membrane and steel shaft of the probe. (F) The in vitro fixed trestle of the microdialysis probe was applied to store and clean probes. (G) Four syringe liquid delivery of the syringe pump. (H, I) The stainless-steel swivels and multi-channel swivel controller on the system for freely moving animals. (J) Two-channel refrigerated fraction collector. (K) Obtained rat HECF by microdialysis. (L) Free movement tank for rats. (M) Related components of the microdialysis sampling system-. Please click here to view a larger version of this figure.
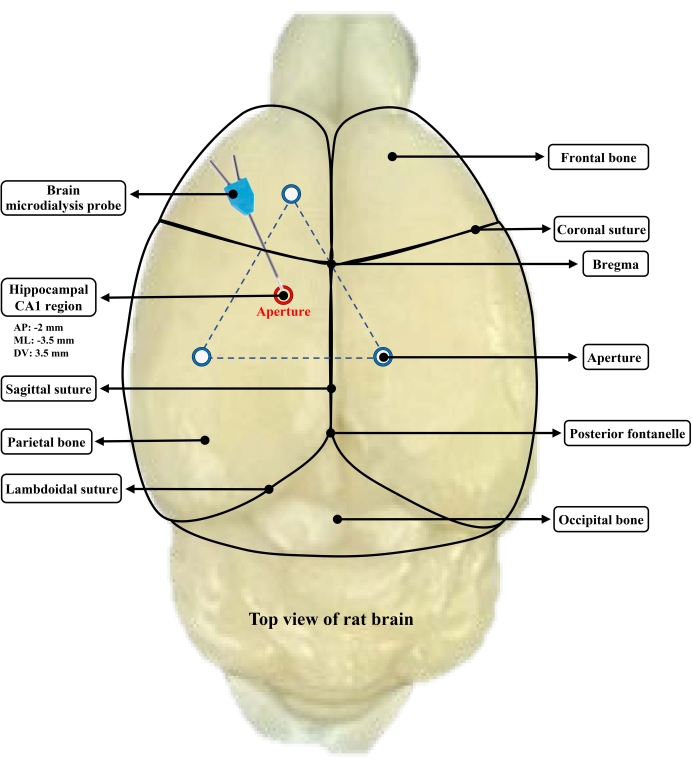
Figure 2: Schematic diagram of the brain microdialysis probe embedded in the hippocampus region of rat brain. Three apertures form a triangle, and the probe orifice is centrally located. Please click here to view a larger version of this figure.
| Parameters items | Value |
| Perfusion rate | 1 μL/min |
| Sampling rate | 1 μL/min |
| Sampling temperature | 4 °C |
Table 1: Set parameters for the microdialysis cerebrospinal fluid sampling system.
Discussion
The pathogenesis of CNS diseases is still not fully understood, which hinders the development of new therapies and drugs. Studies have shown that most CNS diseases are closely related to hippocampal lesions20,21,22. The proposed brain microdialysis technique can target specific regions of the brain, especially the hippocampus, which makes it stand out from the traditional approach of collecting HECF. Probes are placed in the CA1 region of the rat brain through implantation surgery to separate molecules of specific size by passive diffusion of an artificial membrane. The implantation of the probe is a crucial step where any damage to the probe and any local damage of brain tissue, such as protein, aggregates23, which may have been caused by probe implantation, will lead to failure of the experiment or an increase in the inaccuracy of measurement. Therefore, it is essential to check the integrity of the probe and give the animal a proper recovery period after microdialysis probe implantation surgery.
In recent years, the use of ethnomedicine to treat brain diseases has been growing24,25. The traditional method of obtaining cerebrospinal fluid and interstitial fluid in the brain is mostly a one-time event with a high probability of blood contamination8,9. Most importantly, it is impossible to observe the dynamic changes of drugs and their metabolites in the body. As an online sampling technique for awake organisms, brain microdialysis has the characteristics of being in vivo, minimally invasive, a small sample size, real-time, and dynamic, which makes up for the defects of the traditional sampling methods26. Combined with modern analysis and detection technology, qualitative and quantitative analysis of disease factors and drug components can be conducted more accurately27. In general, it is of great significance to introduce brain microdialysis for the study of brain diseases and reveal the action mechanism of ethnomedicine.
The in vitro microdialysis sampling technique of HECF can be applied to the prevention and treatment of CNS diseases by drugs. Secondary brain injuries involving changes in HECF compositions are the main cause of increased mortality of ischemic hypoxic brain injury and traumatic brain injury. In response, analysis of HECF based on brain microdialysis technology can dynamically diagnose early biomarkers of these CNS diseases to reduce morbidity and mortality, as well as improve prognosis28,29. After treatment, drug concentration in brain tissue is routinely determined by measuring homogenized whole brain tissue during preclinical studies, but direct observation of concentration at the specific brain regions cannot be performed. To overcome this, drug concentrations and pathological markers in specific brain regions can be quantitatively analyzed in combination with brain microdialysis sampling techniques30. Particularly for multi-component ethnic herbs, chemical analysis based on brain microdialysis sampling could focus and uncover the mystery of composition-brain region-mechanisms in the treatment of CNS diseases31,32. In addition, changes in the color, transparency, and osmotic pressure of the rat HECF may occur in different disease states, such as cerebral hemorrhage, brain tumors, and meningitis. Using HPLC or mass spectrometry, researchers can determine the changes in HECF composition in different encephalopathies.
In general, brain microdialysis sampling technology can facilitate the investigation of the pathological mechanism of CNS diseases and the development of new drugs. However, further constraints that need to be overcome for effective application of the system include the damage to surrounding tissue after insertion of the microdialysis probe in the targeted region of the brain, the possibility of BBB destruction, and limited mass transfer across the membrane14,33,34. In conclusion, brain microdialysis technology has broad application prospects in the exploration of CNS pathogenesis and the development of new drugs.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82104533), the Science & Technology Department of Sichuan Province (2021YJ0175), and the China Postdoctoral Science Foundation (2020M683273). The authors would like to thank Mr. Yuncheng Hong, a senior equipment engineer at Tri-Angels D&H Trading Pte. Ltd. (Singapore) for providing technical services for the microdialysis technique.
Materials
| Air-drying oven | Suzhou Great Electronic Equipment Co., Ltd | GHG-9240A | |
| Animal anesthesia system | Rayward Life Technology Co., Ltd | R500IE | |
| Animal temperature maintainer | Rayward Life Technology Co., Ltd | 69020 | |
| Artificial cerebrospinal fluid | Beijing leagene biotech. Co., Ltd | CZ0522 | |
| Brain microdialysis probe | CMA Microdialysis AB | T56518 | |
| Catheter | CMA Microdialysis AB | T56518 | |
| Covance infusion harness | Instech Laboratories, Inc. | CIH95 | |
| Denture base resins | Shanghai Eryi Zhang Jiang Biomaterials Co., Ltd | 190732 | |
| Electric cranial drill | Rayward Life Technology Co., Ltd | 78001 | |
| Electric shaver | Rayward Life Technology Co., Ltd | CP-5200 | |
| Free movement tank for animals | CMA Microdialysis AB | CMA120 | |
| Heparin sodium injection | Chengdu Haitong Pharmaceutical Co., Ltd | H51021208 | |
| Iodophor | Sichuan Lekang Pharmaceutical Accessories Co., Ltd | 202201 | |
| Isofluran | Rayward Life Technology Co., Ltd | R510-22 | |
| Microdialysis catheter stylet | CMA Microdialysis AB | 8011205 | |
| Microdialysis collection tube | CMA Microdialysis AB | 7431100 | |
| Microdialysis collector | CMA Microdialysis AB | CMA4004 | |
| Microdialysis fep tubing | CMA Microdialysis AB | 3409501 | |
| Microdialysis in vitro stand | CMA Microdialysis AB | CMA130 | |
| Microdialysis microinjection pump | CMA Microdialysis AB | 788130 | |
| Microdialysis syringe (1.0 mL) | CMA Microdialysis AB | 8309020 | |
| Microdialysis tubing adapter | CMA Microdialysis AB | 3409500 | |
| Non-absorbable surgical sutures | Shanghai Tianqing Biological Materials Co., Ltd | S19004 | |
| Ophthalmic forceps | Rayward Life Technology Co., Ltd | F12016-15 | |
| Osmometer | Löser | OM 807 | |
| Sodium hyaluronate eye drops | URSAPHARM Arzneimittel GmbH | H20150150 | |
| Stereotaxie apparatus | Rayward Life Technology Co., Ltd | 68025 | |
| Surgical scissors | Rayward Life Technology Co., Ltd | S14014-15 | |
| Surgical scissors | Shanghai Bingyu Fluid technology Co., Ltd | BY-103 | |
| Syringe needle | CMA Microdialysis AB | T56518 | |
| Trypsin solution | Boster Biological Technology, Ltd. |
PYG0107 | |
| Ultrasonic cleaner | Guangdong Goote Ultrasonic Co., Ltd | KMH1-240W8101 |
References
- Erkkinen, M. G., Kim, M. O., Geschwind, M. D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harbor Perspectives in Biology. 10 (4), 033118 (2018).
- Salehi, A., Zhang, J. H., Obenaus, A. Response of the cerebral vasculature following traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism. 37 (7), 2320-2339 (2017).
- Kurtzman, R. A. e. m. 3., Caruso, J. L. High-altitude illness death investigation. Academic Forensic Pathology. 8 (1), 83-97 (2018).
- Matsumoto, T., et al. Pharmacokinetic study of Ninjin’yoeito: Absorption and brain distribution of Ninjin’yoeito ingredients in mice. Journal of Ethnopharmacology. 279, 114332 (2021).
- Mahat, M. Y., et al. An improved method of transcutaneous cisterna magna puncture for cerebrospinal fluid sampling in rats. Journal of Neuroscience Methods. 211 (2), 272-279 (2012).
- Ceaglio, N., et al. High performance collection of cerebrospinal fluid in rats: evaluation of erythropoietin penetration after osmotic opening of the blood-brain barrier. Journal of Neuroscience Methods. 219 (1), 70-75 (2013).
- Bothwell, S. W., Janigro, D., Patabendige, A. Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids and Barriers of the CNS. 16 (1), 9 (2019).
- Barthel, L., et al. A step-by-step guide for microsurgical collection of uncontaminated cerebrospinal fluid from rat cisterna magna. Journal of Neuroscience Methods. 352, 109085 (2021).
- Zhao, Y., Yang, Y., Wang, D. X., Wang, J., Gao, W. Y. Cerebrospinal fluid amino acid metabolite signatures of diabetic cognitive dysfunction based on targeted mass spectrometry. Journal of Alzheimer’s Disease. 86 (4), 1655-1665 (2022).
- Lim, N. K., et al. An improved method for collection of cerebrospinal fluid from anesthetized mice. Journal of Visualized Experiments. (133), e56774 (2018).
- Hendrickx, S., et al. A sensitive capillary LC-UV method for the simultaneous analysis of olanzapine, chlorpromazine and their FMO-mediated N-oxidation products in brain microdialysates. Talanta. 162, 268-277 (2017).
- Chefer, V. I., Thompson, A. C., Zapata, A., Shippenberg, T. S. Overview of brain microdialysis. Current Protocols in Neuroscience. , (2009).
- Hammarlund-Udenaes, M. Microdialysis as an important technique in systems pharmacology-a historical and methodological review. The AAPS Journal. 19 (5), 1294-1303 (2017).
- Anderzhanova, E., Wotjak, C. T. Brain microdialysis and its applications in experimental neurochemistry. Cell and Tissue Research. 354 (1), 27-39 (2013).
- Mohammadi, A., Rashidi, E., Amooeian, V. G. Brain, blood, cerebrospinal fluid, and serum biomarkers in schizophrenia. Psychiatry Research. 265, 25-38 (2018).
- Lashley, T., et al. Molecular biomarkers of Alzheimer’s disease: progress and prospects. Disease Models & Mechanisms. 11 (5), 031781 (2018).
- Kawata, K., Tierney, R., Langford, D. Blood and cerebrospinal fluid biomarkers. Handbook of Clinical Neurology. 158, 217-233 (2018).
- Zhao, Q. P., et al. Protective effects of dehydrocostuslactone on rat hippocampal slice injury induced by oxygen-glucose deprivation/reoxygenation. International Journal of Molecular Medicine. 42 (2), 1190-1198 (2018).
- Wang, X. B. . Protective effects of dehydrocostuslactone on oxygen-glucose deprivation injury in rat hippocampal slices. , (2017).
- Coimbra-Costa, D., Alva, N., Duran, M., Carbonell, T., Rama, R. Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biology. 12, 216-225 (2017).
- Liu, H. Y., Chou, K. H., Chen, W. T. Migraine and the Hippocampus. Current Pain and Headache Reports. 22 (2), 13 (2018).
- Toda, T., Parylak, S. L., Linker, S. B., Gage, F. H. The role of adult hippocampal neurogenesis in brain health and disease. Molecular Psychiatry. 24 (1), 67-87 (2019).
- Wang, P., Lo Cascio, F., Gao, J., Kayed, R., Huang, X. F., F, X. Binding and neurotoxicity mitigation of toxic tau oligomers by synthetic heparin like oligosaccharides. Chemical Communications. 54 (72), 10120-10123 (2018).
- Han, J. Y., Li, Q., Ma, Z. Z., Fan, J. Y. Effects and mechanisms of compound Chinese medicine and major ingredients on microcirculatory dysfunction and organ injury induced by ischemia/reperfusion. Pharmacology & Therapeutics. 177, 146-173 (2017).
- Peng, T. M., et al. Anti-inflammatory effects of traditional Chinese medicines on preclinical in vivo models of brain ischemia-reperfusion-injury: Prospects for neuroprotective drug discovery and therapy. Frontiers in Pharmacology. 10, 204 (2019).
- König, M., Thinnes, A., Klein, J. Microdialysis and its use in behavioural studies: Focus on acetylcholine. Journal of Neuroscience Methods. 300, 206-215 (2018).
- Liu, M. Z., Wang, P., Yu, X. M., Dong, G. C., Yue, J. Intracerebral microdialysis coupled to LC-MS/MS for the determination tramadol and its major pharmacologically active metabolite O-desmethyltramadol in rat brain microdialysates. Drug Testing and Analysis. 9 (8), 1243-1250 (2017).
- de Lima Oliveira, M., et al. Cerebral microdialysis in traumatic brain injury and subarachnoid hemorrhage: state of the art. Neurocritical Care. 21 (1), 152-162 (2014).
- Amiridze, N., Dang, Y., Brown, O. R. Hydroxyl radicals detected via brain microdialysis in rats breathing air and during hyperbaric oxygen convulsions. Redox Report. 4 (4), 165-170 (1999).
- Chang, H. Y., Morrow, K., Bonacquisti, E., Zhang, W., Shah, D. K. Antibody pharmacokinetics in rat brain determined using microdialysis. MABS. 10 (6), 843-853 (2018).
- Wan, H. Y., et al. Pharmacokinetics of seven major active components of Mahuang decoction in rat blood and brain by LC-MS/MS coupled to microdialysis sampling. Naunyn-Schmiedeberg’s Archives of Pharmacology. 393 (8), 1559-1571 (2020).
- Zheng, H. Z., et al. Pharmacokinetic analysis of Huangqi Guizhi Wuwu decoction on blood and brain tissue in rats with normal and cerebral ischemia-reperfusion Injury by microdialysis with HPLC-MS/MS. Drug Design Development and Therapy. 14, 2877-2888 (2020).
- Bongaerts, J., et al. Sensitive targeted methods for brain metabolomic studies in microdialysis samples. Journal of Pharmaceutical and Biomedical Analysis. 161, 192-205 (2018).
- Zhang, Y. Q., Jiang, N., Yetisen, A. K. Brain neurochemical monitoring. Biosensors and Bioelectronics. 189, 113351 (2021).

