Detection of Polyfunctional T Cells in Children Vaccinated with Japanese Encephalitis Vaccine via the Flow Cytometry Technique
Summary
The present protocol combines ex vivo stimulation and flow cytometry to analyze polyfunctional T cell (TPF) profiles in peripheral blood mononuclear cells (PBMCs) within Japanese encephalitis virus (JEV)-vaccinated children. The detection method and flow cytometry color scheme of JEV-specific TPFs were tested to provide a reference for similar studies.
Abstract
T cell-mediated immunity plays an important role in controlling flavivirus infection, either after vaccination or after natural infection. The “quality” of a T cell needs to be assessed by function, and higher function is associated with more powerful immune protection. T cells that can simultaneously produce two or more cytokines or chemokines at the single-cell level are called polyfunctional T cells (TPFs), which mediate immune responses through a variety of molecular mechanisms to express degranulation markers (CD107a) and secrete interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-2, or macrophage inflammatory protein (MIP)-1α. There is increasing evidence that TPFs are closely related to the maintenance of long-term immune memory and protection and that their increased proportion is an important marker of protective immunity and is important in the effective control of viral infection and reactivation. This evaluation applies not only to specific immune responses but also to the assessment of cross-reactive immune responses. Here, taking the Japanese encephalitis virus (JEV) as an example, the detection method and flow cytometry color scheme of JEV-specific TPFs produced by peripheral blood mononuclear cells of children vaccinated against Japanese encephalitis were tested to provide a reference for similar studies.
Introduction
Japanese encephalitis virus (JEV) is an important mosquito-borne virus belonging to the genus Flavivirus within the Flaviviridae family1. Many Asia-Pacific countries have long faced enormous public health challenges due to the huge disease burden caused by Japanese encephalitis (JE), but this has improved dramatically with the increasing availability of various types of vaccinations2. Adaptive protective immune responses evoked by natural infection or vaccination contribute to the prevention and antiviral regulation. Humoral immunity and cell-mediated immunity are classified as adaptive immunity, and the induction of the former has always been regarded as a key strategy in vaccine design, albeit with relatively limited understanding in the past3. However, the role of T cell-mediated immunity in limiting flavivirus dissemination and virus clearance has been increasingly focused on and extensively studied4. Furthermore, T cell immunity is not only indispensable in JEV-specific antiviral responses but also plays a prominent role in cross-protection from secondary infection with heterologous flaviviruses, which has been demonstrated in previous studies5. It is speculated that this effect may bypass potential antibody-mediated enhancement effects in infection5. Of note, such cross-reactive T cell immunity is important, especially in the absence of vaccines and antiviral drugs against flaviviruses. Although many studies have been performed to determine the contribution of T cells in JEV infection with respect to CD4+ and CD8+ T cells6,7, the respective lineages secreting cytokines and their functional diversification remain undetermined, which means the elucidation of the exact functions of helper and killer T cells is hindered.
The scale of their antiviral defenses determines the quality of T cell responses. CD4+ or CD8+ T cells that can compatibly confer two or more functions, including cytokine secretion and degranulation, are characterized as polyfunctional T cells (TPFs) upon specific stimulation at the single-cell level8. CD4+ T cells that produce single or multiple cytokines may have various effects and immune memories. For example, IL-2+ IFN-γ+ CD4+ T cells are more likely to form a long-term effective protective response than IL-2+ CD4+ T cells9, which can be used as an important parameter in evaluating the vaccination effect. The frequency of IL-2+ IFN-γ+ CD4+ T cells is increased in patients with long-term non-progression of acquired immune deficiency syndrome (AIDS), while CD4+ T cells in patients with AIDS progression are more inclined to produce IFN-γ alone due to the promoting effect of IL-2 on T cell proliferation10. Furthermore, a subset of IL-2+ IFN-γ+ TNF-α+ was shown to survive long-term in vivo and synergistically promote the killing function11. Although CD8+ T cells are more likely to exhibit cytotoxic activity, some CD4+ T cells are also equipped with cytotoxic activity as an indirectly detected expression of surface CD107a molecules12. In addition, certain T cell subsets express the chemokine MIP-1α, which is often secreted by monocytes to participate in T cell-mediated neutrophil recruitment13. Similarly, CD8+ TPFs can also be used to characterize the versatility of the above markers. Studies have shown that the prime-boost strategy can effectively induce a prolonged period of TPF protective effects13, which can enhance the protection elicited by vaccination. A central feature in examining the immune system is the ability of memory T cells to facilitate stronger, faster, and more effective responses to secondary viral challenges than naïve T cells. Effector memory T cells (TEM) and central memory T cells (TCM) are important T cell subsets that are often differentiated by the composite expression of CD27/CD45RO or CCR7/CD45RA14. TCM (CD27+ CD45RO+ or CCR7+ CD45RA–) tends to localize in secondary lymphoid tissues, while TEM (CD27– CD45RO+ or CCR7– CD45RA–) localizes in lymphoid and peripheral tissues15,16. TEM provides immediate but not sustained defense, whereas TCM sustains the response by proliferating in the secondary lymphoid organs and generating new effectors17. Thus, given that memory cells can mediate specific and efficient recall responses to viruses, questions arise about the contribution of this subset of polyfunctions.
With the development of flow cytometry technology, it has become common to simultaneously detect markers of more than 10 clusters, phenotypes, and differentiation antigens, which is beneficial to more abundantly annotate the functional immunological features on individual T cells to reduce misinterpretation and difficulties in understanding of T cell phenotypes. This study used ex vivo stimulation and flow cytometry to analyze TPF profiles in peripheral blood mononuclear cells (PBMCs) within JEV-vaccinated children. Applying this approach, the understanding of short- and long-term JEV-specific and even cross-reactive T cell immunity induced by vaccination will be expanded.
Protocol
Ethical approval for the present study was obtained by the Ethics Committee of Beijing Children's Hospital, Capital Medical University (Approval Number: 2020-k-85). Volunteers were recruited from Beijing Children's Hospital, Capital Medical University. Peripheral venous blood samples were obtained from apparently healthy children (2 years old) who had previously received a prime and boosted vaccination with live-attenuated JE SA14-14-2 vaccine for less than half a year (JE-vaccinated children, n = 5) and unvaccinated children (6 months old, n = 5). Informed consent of human subjects was waived as only the residual samples, after being clinically tested, were used in this study. To protect the privacy of the volunteers, all data were fully anonymized and de-identified.
1. Isolation of PBMCs from peripheral venous blood
- Collect peripheral venous blood samples (2 mL) from JE-vaccinated and unvaccinated children in EDTA-K2-anticoagulated tubes (see Table of Materials) by the standard venipuncture technique18.
- Add 2 mL of phosphate-buffered saline (1x PBS) to dilute the peripheral blood.
NOTE: The process is handled as soon as possible to maintain maximum survivability. - Add 4 mL of the density gradient medium (see Table of Materials) to a 15 mL centrifuge tube, and slowly transfer the diluted blood to the upper layer of the separation medium.
NOTE: Do not mix the blood with the separation medium or destroy the contact surface formed by the two liquids; otherwise, the separation effect will not be achieved. - Centrifuge at 800 × g for 20 min at room temperature. Observe the significant layers after centrifugation.
- Transfer the PBMCs (the middle layer) to another 15 mL centrifuge tube, and wash the PBMCs with 10 mL of RPMI-1640 medium containing 10% fetal bovine serum (FBS, see Table of Materials).
- Centrifuge at 800 × g for 10 min at room temperature and discard the supernatant with a pipette carefully.
- Resuspend the PBMCs with 1 mL of RPMI-1640 medium containing 10% FBS and count the cells with a trypan blue-based automated counter (see Table of Materials).
2. Stimulation of PBMCs by inactivated JEV particles to induce cytokine expression
- Set two subgroups of the JEV stimulation and control groups in the JE-vaccinated and unvaccinated samples, respectively.
- Adjust the number of PBMCs to 2 × 106 cells/mL with RPMI-1640 medium containing 10% FBS, and seed the PBMCs in 24-well plates (2 × 106 cells/1 mL medium per well); inoculate three wells for each group.
- Stimulate the PBMCs of the JEV stimulation group with concentrated inactivated JEV particles5 (2 × 105 PFU) for 16 h at 37 °C in the presence of monoclonal antibodies CD28 (1 µg/mL, 1:1,000) and CD49d (1 µg/mL), GolgiPlug (1 µg/mL, 1:1,000), and monensin (1 µg/mL, 1:1,000). For surface staining, use BV605-anti-CD107a (1 µg/mL, 1:1,000) (see Table of Materials).
NOTE: The JEV was inactivated by UV irradiation as described previously19. - Stimulate the PBMCs of the control group without concentrated virus particles for 16 h at 37 °C in the presence of monoclonal antibodies CD28 (1 µg/mL, 1:1,000) and CD49d (1 µg/mL, 1:1,000), GolgiPlug (1 µg/mL, 1:1,000), and monensin (1 µg/mL, 1:1,000). Stain the surface with BV605-anti-CD107a (1 µg/mL, 1:1,000).
3. Ex vivo intracellular staining
- Collect the cell suspension from each group in a 1.5 mL microcentrifuge tube, centrifuge at 500 × g for 5 min at room temperature, and remove the supernatant with a pipette.
- Resuspend the cells in 1 mL of 1x PBS, add fixable viability dye (1 µL/mL, 1:1,000, see Table of Materials) to the cell suspension, and incubate for 10 min at room temperature in the dark. Centrifuge at 500 × g (at room temperature) for 5 min and remove the supernatant.
- Resuspend the cells in 1 mL of 1x PBS. Centrifuge at 500 × g (at room temperature) for 5 min. Discard the supernatant carefully.
- Perform cell surface marker staining.
- Resuspend the cells in 100 µL of 1x PBS, and add 2 µL of each surface markers antibody (BV650-anti-CD3, BUV395-anti-CD4, BV421-anti-CD8, BUV737-anti-CD27, and BV480-anti-CD45RO, dilution factor: 1:50, see Table of Materials) to the cell suspension in each tube.
NOTE: The color fluorescent antibody staining protocol is shown in Table 1. - Incubate the tubes (step 3.4.1) for 30 min at room temperature, and protect them from light. Centrifuge at 500 × g for 5 min and remove the supernatant.
NOTE: The antibody of BUV737-anti-CD27 and BV480-anti-CD45RO can be replaced with BUV737-anti-CCR7 and BV480-anti-CD45RA as the annotation of the memory T cells.
- Resuspend the cells in 100 µL of 1x PBS, and add 2 µL of each surface markers antibody (BV650-anti-CD3, BUV395-anti-CD4, BV421-anti-CD8, BUV737-anti-CD27, and BV480-anti-CD45RO, dilution factor: 1:50, see Table of Materials) to the cell suspension in each tube.
- Resuspend the cells in 1 mL of 1x PBS. Centrifuge at 500 × g for 5 min at room temperature. Discard the supernatant carefully.
- Perform fixation and membrane breaking.
- Resuspend the cells with 500 µL of membrane-breaking fixative solution (see Table of Materials) and fix the cells for 20 min in the dark at room temperature. Centrifuge at 500 × g for 5 min and remove the supernatant.
- Resuspend the cells in 1 mL of 1x PBS. Centrifuge for 5 min at 500 × g at room temperature and remove the supernatant carefully.
- Perform intracellular cytokine staining.
- Resuspend the cells in 100 µL of 1x PBS, and add 2 µL of each cytokine antibody (FITC-anti-IFN-γ, PE-anti-TNF-α, BV785-anti-IL-2, and APC-anti-MIP-1α, dilution factor: 1:50, see Table of Materials) to the cell suspension in each tube.
NOTE: The volumes and information on fluorophore conjugated antibodies are shown in Table 1. - Incubate the tubes (step 3.8.1) for 30 min at room temperature in the dark. Centrifuge at 500 × g for 5 min and remove the supernatant.
- Resuspend the cells in 100 µL of 1x PBS, and add 2 µL of each cytokine antibody (FITC-anti-IFN-γ, PE-anti-TNF-α, BV785-anti-IL-2, and APC-anti-MIP-1α, dilution factor: 1:50, see Table of Materials) to the cell suspension in each tube.
- Resuspend the cells in 1 mL of 1x PBS. Centrifuge at 500 × g for 5 min at room temperature, and remove the supernatant carefully. Add 500 µL of 1x PBS to resuspend the cells.
4. Flow cytometry set-up
- Isolate the PBMC sample alone following the procedures described in step 1 as the control sample. Divide the cell suspension into 12 equal parts in 1.5 mL microcentrifuge tubes (100 µL/tube) as mentioned below.
- Set up unstained, APC-Cy7-live/dead fixable stained, BV650-anti-CD3 single-stained, BUV395-anti-CD4 single-stained, BV421-anti-CD8 single-stained, BUV737-anti-CD27 single-stained, BV480-anti-CD45RO stained, BV605-anti-CD107a, FITC-anti-IFN-γ stained, PE-anti-TNF-α single-stained, BV785-anti-IL-2 single-stained, and APC-anti-MIP-1α single-stained samples.
- Add the cell surface markers and intracellular cytokine staining as described in step 3. For each single-staining sample, only add one of the fluorophores from the staining step. Add 500 µL of 1x PBS to resuspend the cells and vortex with low velocity.
- Using an unstained sample, adjust the forward scatter (FSC), side scatter (SSC), and different fluorescent dye voltages.
NOTE: For the present study, the following voltages were set. FSC: 440 V, SSC: 233 V, BV650: 575 V, APC-Cy7: 449 V, BUV395: 500 V, BV421: 402 V, BUV737: 585 V, BV480: 444 V, BV605: 457 V, FITC: 475 V, PE: 469 V, APC: 623 V, and BV785: 725 V. - Using the single-staining samples, adjust the flow cytometry compensation to eliminate the contamination signals between the different fluorophores.
NOTE: The compensation parameters are shown in Table 2.
5. Gating strategy and data analysis
NOTE: In the present study, for this analysis, the central memory T cells (TCM of CD8+ or CD4+ T cells as CD27+ CD45RO+ or CCR7+ CD45RA– and the effector memory T cells (TEM) of CD8+ or CD4+ T cells as CD27– CD45RO+ or CCR7– CD45RA– were defined respectively16.
- Draw a polygon gate through the FSC-area (FSC-A)/SSC-area (SSC-A) dot plot to select the intact lymphocyte population while excluding the debris (Figure 1A).
- Draw a rectangular gate through the FSC-A/FSC-width (FSC-W) dot plot to select the single cells (Figure 1B).
- Draw a rectangular gate through the live/dead/SSC-A dot plot to select the live cells (Figure 1C).
- Draw a rectangular gate through the CD3/SSC-A dot plot to identify the CD3+ T cells (Figure 1D).
- Draw a quad gate through the CD4/CD8 dot plot to identify the CD4+ or CD8+ T cells (Figure 1E).
- Draw a quad gate through the CD45RO/CD27 dot plot to subdivide the CD4+ or CD8+ T cells into TCM (CD27+ CD45RO+) and TEM (CD27– CD45RO+) (Figure 1F-I).
- Draw the gates of CD107a, IFN-γ, TNF-α, IL-2, and MIP-1α from the TCM or TEM of the CD8+ or CD4+ T cells to determine the frequency of different response patterns, respectively.
- Load the samples onto the cytometry sequentially. Use a stopping gate20 to acquire 1 × 106 lymphocytes.
NOTE: The individual user can collect more than 1 × 106 cells. This number was a compromise between the time taken and the collection of enough cells to provide meaningful results.
Representative Results
Figure 1 shows the gating strategy used to divide the TCM or TEM of CD8+ or CD4+ T cells from a representative JEV stimulation group of JE-vaccinated children. The FSC-A/SSC-A dot plot is used to identify lymphocytes, and the FSC-A/FSC-W dot plot is used to identify single cells. Viable cells are selected on the live/dead/SSC-A dot plot. The CD3/SSC-A dot plot is used to identify the CD3+ T cells. The CD4/CD8 dot plot is used to identify the CD3+ CD4+ and CD3+ CD8+ T cells, respectively. The TCM (CD27+ CD45RO+) and TEM (CD27– CD45RO+) of CD8+ or CD4+ T cells are selected separately on the CD45RO/CD27 dot plot. Cells with the phenotype of CD107a+, IL-2+, TNF-α+, IFN-γ+, and MIP-1α+ from CD8+ TCM (Figure 1F), CD8+ TEM (Figure 1G), CD4+ TCM (Figure 1H), and CD4+ TEM (Figure 1I) are selected separately by drawing the corresponding rectangular gate.
The polyfunctional characterization of central and effector memory CD4+ and CD8+ T cell responses (including degranulation, cytokines, and chemokines) to JEV in JE-vaccinated and unvaccinated children are shown in Table 3. These results indicate that increased levels of CD107a, IFN-γ, TNF-α, and IL-2 were detected in the CD8+ TCM cells of the vaccinated children after JEV stimulation compared to those in unvaccinated children. The level of MIP-1α did not differ between the two groups. TEM cells are thought to exert antiviral effects directly upon restimulation with JEV. Higher levels of CD107a and IFN-γ were detected in the CD8+ TEM cells of the vaccinated group under JEV stimulation compared with the unvaccinated group. However, TNF-α, IL-2, and MIP-1α were not significantly elevated in those positive cells.
The JEV antigen successfully induced higher levels of CD107a, IFN-γ, TNF-α, and MIP-1α in the CD4+ TCM cells of the vaccinated group compared with the unvaccinated group. However, the proportion of IL-2+ cells did not differ between the two groups under JEV stimulation. The proportion of the CD107a+, IFN-γ+, and TNF-α+ subsets of CD4+ TEM cells in the vaccinated group was higher in the presence of JEV than in the unvaccinated group.
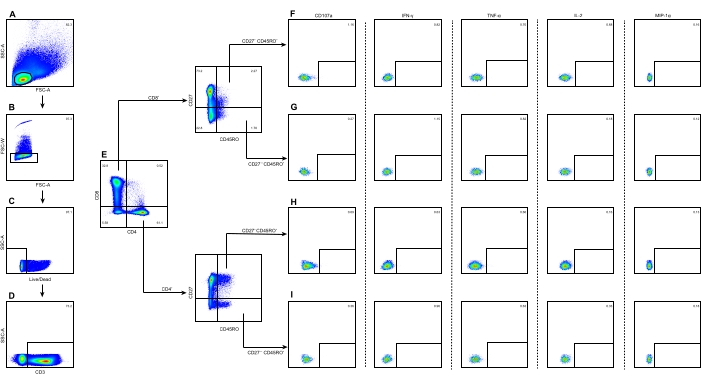
Figure 1: Illustration of the gating strategy to identify the TCM or TEM of CD8+ or CD4+ T cells and their subsets. (A) Lymphocytes were identified using the FSC-A/SSC-A dot plot. (B) Single cells were identified using the FSC-A/FSC-W dot plot. (C) Live cells were identified using the live/dead/SSC-A dot plot. (D) CD3+ T cells were identified using the CD3/SSC-A dot plot. (E) CD3+ CD4+ T and CD3+ CD8+ T cells were identified using the CD4/CD8 dot plot. (F) CD8+ TCM with the phenotype of CD107a+, IL-2+, TNF-α+, IFN-γ+, and MIP-1α+were subdivided separately. (G) CD8+ TEM with the five phenotypes were subdivided separately. (H) CD4+ TCM with the five phenotypes were subdivided separately. (I) CD4+ TEM with the five phenotypes were subdivided separately. The numbers on each panel represent the percentage of cells in the gates. The color coding represents the frequency of occurrence of the cells, where red is the highest frequency and blue is the lowest. Abbreviations: SSC-A = side scatter-area; FSC-A = forward scatter-area; FSC-W = forward scatter-width. Please click here to view a larger version of this figure.
| Antibody Target | Conjugated Fluorophore | Dosage | Clone | Isotype | Excitation laser line | Ex-Max (nm) | Em-Max (nm) |
| CD3 | BV650 | 2 µL | SK7 | Mouse IgG1, κ | Violet | 407 | 650 |
| CD4 | BUV395 | 2 µL | SK3 | Mouse IgG1, κ | UV | 348 | 39 |
| CD8 | BV421 | 2 µL | SK1 | Mouse IgG1, κ | Violet | 407 | 421 |
| CD27 | BUV737 | 2 µL | L128 | Mouse IgG1, κ | UV | 350 | 737 |
| CD45RO | BV480 | 2 µL | UCHL1 | Mouse IgG2a, κ | Violet | 436 | 478 |
| CCR7 | BUV737 | 2 µL | 3D12 | Rat IgG2a, κ | UV | 350 | 737 |
| CD45RA | BV480 | 2 µL | HI100 | Mouse IgG2b, κ | Violet | 436 | 478 |
| CD107a | BV605 | 2 µL | H4A3 | Mouse IgG1, κ | Violet | 407 | 602 |
| IL-2 | BV785 | 2 µL | MQ1-17H12 | Rat IgG2a, κ | Violet | 407 | 786 |
| IFN-γ | FITC | 2 µL | 4S.B3 | Mouse IgG1, κ | Blue | 494 | 520 |
| TNF-α | PE | 2 µL | MAb11 | Mouse IgG1, κ | Yellow-Green | 496, 564 | 578 |
| MIP-1α | APC | 2 µL | 11A3 | Mouse IgG2a, κ | Red | 650 | 660 |
| Zombie NIR | APC-Cy7 | 1 µL | – | – | Red | 650 | 779 |
Table 1: The color fluorescent antibody staining protocol for flow cytometric analyses. Abbreviations: BV = brilliant violet; BUV = brilliant ultraviolet; FITC = fluorescein isothiocyanate; PE = phycoerythrin; APC = allophycocyanin.
| Compensation reference of flow cytometry (%) | ||||||||||||
| FITC | APC | APC-Cy7 | BV421 | BV480 | BV605 | BV560 | BV785 | BUV395 | BUV737 | PE | ||
| FITC | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| APC | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| APC-Cy7 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 65 | 0 | 44 | 0 | |
| BV421 | 0 | 0.6 | 0 | 100 | 10.5 | 3 | 15 | 3 | 0.4 | 0 | 0 | |
| BV480 | 3 | 0 | 0 | 46 | 100 | 19 | 0 | 6.1 | 0 | 0 | 0 | |
| BV605 | 0 | 0 | 0 | 3.6 | 0 | 100 | 94 | 10 | 0 | 9 | 14 | |
| BV560 | 0 | 5.5001 | 0 | 2 | 1 | 7.1 | 100 | 9 | 0 | 10 | 0 | |
| BV785 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 30 | 0 | |
| BUV395 | 0 | 0 | 0 | 1.7 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | |
| BUV737 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 3.7001 | 3 | 100 | 0 | |
| PE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | |
Table 2: The parameters of compensation for flow cytometric analyses. Abbreviations: BV = brilliant violet; BUV = brilliant ultraviolet; FITC = fluorescein isothiocyanate; PE = phycoerythrin; APC = allophycocyanin.
| Group of TPF | Polyfunctional characterization (%) | |||||||
| Stimulus | CD107a | IFN-γ | TNF-α | IL-2 | MIP-1α | |||
| CD8+ T cells | TCM | Vaccinated | JEV | 1.50±0.48 | 1.60±0.69 | 0.66±0.32 | 0.54±0.27 | 0.16±0.11 |
| Ctrl | 0.51±0.38 | 0.31±0.13 | 0.28±0.13 | 0.37±0.20 | 0.02±0.05 | |||
| Unvaccinated | JEV | 0.38±0.19 | 0.20±0.03 | 0.19±0.09 | 0.16±0.04 | 0.19±0.09 | ||
| Ctrl | 0.50±0.28 | 0.27±0.09 | 0.19±0.05 | 0.23±0.14 | 0.08±0.11 | |||
| P value* | – | 0.00 | 0.00 | 0.02 | 0.01 | 0.70 | ||
| TEM | Vaccinated | JEV | 1.47±0.58 | 1.21±0.22 | 0.49±0.36 | 0.33±0.31 | 0.24±0.17 | |
| Ctrl | 0.82±0.48 | 0.39±0.15 | 0.16±0.18 | 0.23±0.256 | 0.09±0.21 | |||
| Unvaccinated | JEV | 0.41±0.25 | 0.14±0.09 | 0.15±0.11 | 0.20±0.07 | 0.15±0.13 | ||
| Ctrl | 0.34±0.13 | 0.21±0.15 | 0.17±0.10 | 0.19±0.19 | 0.01±0.02 | |||
| P value* | – | 0.01 | 0.00 | 0.08 | 0.13 | 0.36 | ||
| CD4+ T cells | TCM | Vaccinated | JEV | 1.55±0.43 | 1.16±0.24 | 0.57±0.25 | 0.29±0.18 | 0.19±0.07 |
| Ctrl | 0.44±0.27 | 0.26±0.20 | 0.15±0.06 | 0.12±0.06 | 0.04±0.07 | |||
| Unvaccinated | JEV | 0.28±0.08 | 0.21±0.08 | 0.17±0.03 | 0.21±0.16 | 0.10±0.03 | ||
| Ctrl | 0.31±0.13 | 0.26±0.06 | 0.14±0.04 | 0.25±0.13 | 0.04±0.04 | |||
| P value* | – | 0.00 | 0.00 | 0.01 | 0.47 | 0.04 | ||
| TEM | Vaccinated | JEV | 1.54±0.58 | 1.33±0.15 | 0.89±0.25 | 0.37±0.22 | 0.21±0.05 | |
| Ctrl | 0.46±0.49 | 0.35±0.30 | 0.13±0.08 | 0.14±0.09 | 0.05±0.11 | |||
| Unvaccinated | JEV | 0.27±0.09 | 0.23±0.10 | 0.30±0.15 | 0.23±0.03 | 0.14±0.06 | ||
| Ctrl | 0.35±0.21 | 0.24±0.14 | 0.19±0.20 | 0.25±0.08 | 0.05±0.07 | |||
| P value* | – | 0.00 | 0.00 | 0.00 | 0.19 | 0.08 | ||
| *Mann–Whitney U-test, PBMCs from vaccinated individual stimulated by JEV vs.PBMCs from unvaccinated individual stimulated by JEV were showed only. | ||||||||
Table 3: Polyfunctional characterization of the TCM and TEM of CD4+ or CD8+ T cell responses to JEV. Vaccinated, n = 5; unvaccinated, n = 5. Results are expressed as mean ± SD. P < 0.05 indicated a significant difference. *PBMCs from vaccinated individuals stimulated by JEV versus PBMCs from unvaccinated individuals stimulated by JEV only are shown. Abbreviations: TPFs = polyfunctional T cells; IFN-γ = interferon-γ; TNF-α = tumor necrosis factor-α; IL-2 = interleukin-2; MIP-1α = macrophage inflammatory protein-1α; TCM = central memory T cells; TEM = effector memory T cells.
Discussion
This protocol represents a feasible flow cytometry-based detection method for TPF profiles in the PBMCs of children vaccinated with the JEV vaccine SA14-14-2. This study used the venous blood PBMCs of both vaccinated and unvaccinated children as research materials. With the stimulation of PBMCs with the JEV antigen, those amplified antigen-specific TPFs can be characterized by multicolor flow cytometry antibody staining. Compared with the conventional enzyme-linked immunospot assay method, flow cytometry's outstanding advantage is presenting the functional features of PBMCs at the single-cell level as diversely as possible, reducing the number of PBMCs required for detection, which is especially suitable for children.
A clear vaccination history, maintained cell viability, the T cell immunogenicity of the stimulator, and compatible color matching strategies are critical steps in this protocol. China has incorporated the vaccination of JEV SA14-14-2 into the national expanded immunization program since 2008 and has included the vaccination history into unified management, which has become available for the accessibility of the vaccine history3. The PBMCs examined in this protocol were experimentally kept on ice to maintain maximum cell viability. The use of specific stimuli has previously been widely demonstrated to be effective4,5,21,22. The color matching strategy is the main constraint in this method, and the usability of this strategy has been presented in the continuous pre-test refinement and instrument parameter matching. In addition, this protocol provided two options for annotating the memory T cells. Through the above configuration and optimization of the experimental parameters, it might be achievable to measure specific and even cross-reactive immunity by assessing the scale and frequency of TPF responses to vaccination by flow cytometry. However, the five representative indicators to characterize TPFs are a limitation of this study; the currently common functional molecules (CD107a, IFN-γ, TNF-α, IL-2, and MIP-1α) can be expanded to detect the spectrum in order to more fully characterize the versatility of TPFs based on the presented protocol.
JEV infection is considered a vaccination-preventable disease in which vaccination-evoked memory T-cell responses may be critical in maintaining long-lasting immune protection, especially as antibody responses wane over time4. As the main totipotent protective component in memory T cells, TPFs should be suggested as a routine test item for vaccine efficacy evaluation and, conversely, guide the design and development of targeted T cell vaccines. In fact, the role of T-cell immunity in flavivirus infection control is increasingly becoming appreciated, and it can even bypass the role of antibodies for better or worse5. The clarification of the TPF profile will undoubtedly further narrow the repertoire of antigenic epitopes, which is bound to reduce the cost of vaccines by improving targeting. The synergistic effects of CD4+ and CD8+ T cells complement each other and are undoubtedly more prominent on TPFs. Therefore, it is proposed that studies on TPFs focus on (1) profile differences between long- and short-term TPFs; (2) the identification of HLA-restricted TPF epitopes; and (3) the exploration and research of more polyfunctional subpopulations.
Identifying TPF cells in the immune control of viral infection had been reported23,24,25. However, the description of the complete detection strategy and process of TPFs has not been well organized. Moreover, this method's color scheme applies to multiple flow cytometer models. The markers may also be adjusted to detect other functional subgroups based on this color matching.
This study provided a TPF detection strategy by combining ex vivo specific stimulation and flow cytometry to analyze TPF subset profiles in the PBMCs of JEV vaccine recipients. The isolated PBMCs were first stimulated with the JEV antigen and then stained with the corresponding fluorescently labeled antibodies, and JEV-specific CD4+ and CD8+ memory TPFs were characterized by flow cytometry. Based on this result, the TPF frequencies of double, triple, quadruple, and quintuple functions can be further calculated to describe TPFs in more detail and more comprehensively. Routine venous blood volumes (2 mL) in children have been shown to be sufficient for TPF studies. Therefore, the detection methods for virus-specific TPFs are expected to be used to explore measures of T cell-mediated adaptive immunity associated with vaccination or infection.
Disclosures
The authors have nothing to disclose.
Acknowledgements
R.W. was supported by National Natural Science Foundation of China (82002130), Beijing Natural Science Foundation of China (7222059). ZD.X. was supported by the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-026).
Materials
| anti-human CD28 | Biolegend | 302934 | Antibody |
| anti-human CD49d | Biolegend | 304339 | Antibody |
| APC anti-human MIP-1α | BD | 551533 | Fluorescent antibody |
| Automated cell counter | BIO RAD | TC20 | Cell count |
| BD FACSymphony A5 | BD | A5 | flow Cytometry |
| BUV395 anti-human CD4 | BD | 563550 | Fluorescent antibody |
| BUV737 anti-human CCR7 | BD | 741786 | Fluorescent antibody |
| BUV737 anti-human CD27 | BD | 612829 | Fluorescent antibody |
| BV421 anti-human CD8 | Biolegend | 344748 | Fluorescent antibody |
| BV480 anti-human CD45RA | BD | 566114 | Fluorescent antibody |
| BV480 anti-human CD45RO | BD | 566143 | Fluorescent antibody |
| BV605 anti-human CD107a | Biolegend | 328634 | Fluorescent antibody |
| BV650 anti-human CD3 | BD | 563999 | Fluorescent antibody |
| BV785 anti-human IL-2 | Biolegend | 500348 | Fluorescent antibody |
| Centrifuge Tube | BD Falcon | BD-35209715 | 15 mL centrifuge tube |
| Cytofix/Cytoperm Fixation/Permeabilization Solution Kit | BD | 554714 | Cell fixation and permeabilization |
| Density gradient medium | Dakewe | DKW-KLSH-0100 | Ficoll-Paque, human lymphocyte separation medium |
| FITC anti-human IFN-γ | Biolegend | 502506 | Fluorescent antibody |
| Gibco Fetal Bovine Serum | Thermo Fisher Scientific | 16000-044 | Fetal Bovine Serum |
| Gibco RPMI-1640 medium | Thermo Fisher Scientific | 22400089 | cell culture medium |
| High-speed centrifuge | Sigma | 3K15 | Cell centrifugation for 15 mL centrifuge tube |
| High-speed centrifuge | Eppendorf | 5424R | Cell centrifugation for 1.5 mL Eppendorf (EP) tube |
| Microcentrifuge tubes | Axygen | MCT-150-C | 1.5 mL microcentrifuge tube |
| PE anti-human TNF-α | Biolegend | 502909 | Fluorescent antibody |
| Phosphate Buffered Saline (PBS) | BI | 02-024-1ACS | PBS |
| Protein Transport Inhibitor (Containing Brefeldin A, GolgiPlug) | BD | 555029 | blocks intracellular protein transport processes |
| Protein Transport Inhibitor (Containing Monensin) | BD | 554724 | blocks intracellular protein transport processes |
| Round-bottom test tube | BD Falcon | 352235 | 5 mL test tube |
| Trypan Blue Staining Cell Viability Assay Kit | Beyotime | C0011 | Trypan Blue Staining |
| Zombie NIR Fixable Viability Dye | Biolegend | 423106 | Dead cell stain |
References
- Vanden Eynde, C., Sohier, C., Matthijs, S., De Regge, N. Japanese encephalitis virus interaction with mosquitoes: A review of vector competence, vector capacity and mosquito immunity. Pathogens. 11 (3), 317 (2022).
- Wang, R., et al. The epidemiology and disease burden of children hospitalized for viral infections within the family Flaviviridae in China: A national cross-sectional study. PLoS Neglected Tropical Diseases. 16 (7), 0010562 (2022).
- Wang, R., et al. Decreases in both the seroprevalence of serum antibodies and seroprotection against Japanese encephalitis virus among vaccinated children. Virologica Sinica. 34 (3), 243-252 (2019).
- Wang, R., et al. Neutralizing antibody rather than cellular immune response is maintained for nearly 20 years among Japanese encephalitis SA14-14-2 vaccinees in an endemic setting. Infection, Genetics and Evolution. 85, 104476 (2020).
- Wang, R., et al. T cell immunity rather than antibody mediates cross-protection against Zika virus infection conferred by a live attenuated Japanese encephalitis SA14-14-2 vaccine. Applied Microbiology and Biotechnology. 104 (15), 6779-6789 (2020).
- Redant, V., Favoreel, H. W., Dallmeier, K., Van Campe, W., De Regge, N. Japanese encephalitis virus persistence in porcine tonsils is associated with a weak induction of the innate immune response, an absence of IFNgamma mRNA expression, and a decreased frequency of CD4(+)CD8(+) double-positive T cells. Frontiers in Cellular and Infection Microbiology. 12, 834888 (2022).
- Jain, N., et al. CD8 T cells protect adult naive mice from JEV-induced morbidity via lytic function. PLoS Neglected Tropical Diseases. 11 (2), 0005329 (2017).
- Khakhum, N., Bharaj, P., Walker, D. H., Torres, A. G., Endsley, J. J. Antigen-specific antibody and polyfunctional T cells generated by respiratory immunization with protective Burkholderia DeltatonB Deltahcp1 live attenuated vaccines. NPJ Vaccines. 6 (1), 72 (2021).
- Weaver, J. M., et al. Increase in IFNgamma(-)IL-2(+) cells in recent human CD4 T cell responses to 2009 pandemic H1N1 influenza. PloS One. 8 (-), 57275 (2013).
- Boaz, M. J., Waters, A., Murad, S., Easterbrook, P. J., Vyakarnam, A. Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. Journal of Immunology. 169 (11), 6376-6385 (2002).
- Gui, L., et al. IL-2, IL-4, IFN-gamma or TNF-alpha enhances BAFF-stimulated cell viability and survival by activating Erk1/2 and S6K1 pathways in neoplastic B-lymphoid cells. Cytokine. 84, 37-46 (2016).
- Terahara, K., et al. Vaccine-induced CD107a+ CD4+ T cells are resistant to depletion following AIDS virus infection. Journal of Virology. 88 (24), 14232-14240 (2014).
- Tanyi, J. L., et al. Personalized cancer vaccine strategy elicits polyfunctional T cells and demonstrates clinical benefits in ovarian cancer. NPJ Vaccines. 6 (1), 36 (2021).
- Ammirati, E., et al. Effector memory T cells are associated with atherosclerosis in humans and animal models. Journal of the American Heart Association. 1 (1), 27-41 (2012).
- Rizk, N. M., Fadel, A., AlShammari, W., Younes, N., Bashah, M. The immunophenotyping changes of peripheral CD4+ T lymphocytes and inflammatory markers of class III obesity subjects after laparoscopic gastric sleeve surgery – A follow-up study. Journal of Inflammation Research. 14, 1743-1757 (2021).
- Zhang, Y., et al. Phenotypic and functional characterizations of CD8(+) T cell populations in malignant pleural effusion. Experimental Cell Research. 417 (1), 113212 (2022).
- Shin, H., Iwasaki, A. Tissue-resident memory T cells. Immunological Reviews. 255 (1), 165-181 (2013).
- Birnie, K. A., Noel, M., Chambers, C. T., Uman, L. S., Parker, J. A. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database of Systematic Reviews. 10 (10), (2018).
- Lin, R. J., Liao, C. L., Lin, Y. L. Replication-incompetent virions of Japanese encephalitis virus trigger neuronal cell death by oxidative stress in a culture system. Journal of General Virology. 85, 521-533 (2004).
- Byford, E., Carr, M., Pinon, L., Ahearne, M. J., Wagner, S. D. Isolation of CD4+ T-cells and analysis of circulating T-follicular helper (cTfh) cell subsets from peripheral blood using 6-color flow cytometry. Journal of Visualized Experiments. (143), e58431 (2019).
- Zheng, X., et al. Immune responses and protective effects against Japanese encephalitis induced by a DNA vaccine encoding the prM/E proteins of the attenuated SA14-14-2 strain. Infection, Genetics and Evolution. 85, 104443 (2020).
- Zheng, X., et al. Complete protection for mice conferred by a DNA vaccine based on the Japanese encephalitis virus P3 strain used to prepare the inactivated vaccine in China. Virology Journal. 17 (1), 126 (2020).
- Lam, J. K. P., et al. Emergence of CD4+ and CD8+ polyfunctional T cell responses against immunodominant lytic and latent EBV antigens in children with primary EBV infection. Frontiers in Microbiology. 9, 416 (2018).
- Meckiff, B. J., et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4(+) T cells in COVID-19. Cell. 183 (5), 1340-1353 (2020).
- Ning, R. J., Xu, X. Q., Chan, K. H., Chiang, A. K. Long-term carriers generate Epstein-Barr virus (EBV)-specific CD4(+) and CD8(+) polyfunctional T-cell responses which show immunodominance hierarchies of EBV proteins. Immunology. 134 (2), 161-171 (2011).

