1,6-酮酯高立体选择性合成离子液体介导:三组分反应启用快速进入低分子量胶凝剂的新型类
Summary
Ionic liquids (ILs) mediate fast, simple and cheap access to 1,6-ketoesters in high diastereoselectivities and good yields. The reaction protocol is robust and the 1,6-ketoesters can be obtained in gram scale after a simple filtration protocol. Moreover, the 1,6-ketoesters are potent gelators in hydrocarbon solvents.
Abstract
In organic chemistry ionic liquids (ILs) have emerged as safe and recyclable reaction solvents. In the presence of a base ILs can be deprotonated to form catalytically active N-Heterocyclic Carbenes (NHCs). Here we have used ILs as precatalysts in the addition of α,β-unsaturated aldehydes to chalcones to form 1,6-ketoesters, incorporating an anti-diphenyl moiety in a highly stereoselective fashion. The reaction has a broad substrate scope and several functional groups and heteroaromatics can be integrated into the ketoester backbone in generally good yields with maintained stereoselectivity. The reaction protocol is robust and scalable. The starting materials are inexpensive and the products can be obtained after simple filtration, avoiding solvent-demanding chromatography. Furthermore, the IL can be recycled up to 5 times without any loss of reactivity. Moreover, the 1,6-ketoester end product is a potent gelator in several hydrocarbon based solvents. The method enables rapid access to and evaluation of a new class of low molecular weight gelators (LMWGs) from recyclable and inexpensive starting materials.
Introduction
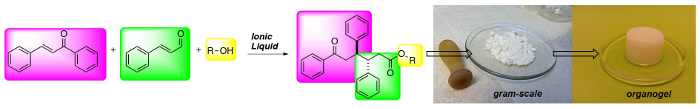
(以上)的1,6-酮酯三组分合成:一类新的低分子量胶凝剂。
离子液体(离子液体)具有高稳定性,低挥发性,不可燃性,并因此呈现关注,因为安全的反应介质和理想的溶剂进行回收。1-3二烷基咪唑鎓是一个特定类型的离子液体,在碱的存在下可以去质子化,以使一个N-杂环卡宾(NHC)。4在有机催化的领域中,的NHCs,根据不同的反应路径操作时,已经被广泛的使用在广泛的通用反应。5-11
尽管如此,ILS和CC键formin之间的连接摹NHC催化相对未开发的。尽管如此,从的NHCs离子液体衍生已经报道了催化CC键形成反应,如苯偶姻缩合和施德达反应。12-22。例如,Davis等人已经表明,从N-烷基thiazoliums衍生离子液体作为在预催化剂形成苯甲醛安息香12
最近,Chen和同事扩展使用基于IL咪唑这个概念,1-乙基-3-甲基咪唑鎓乙酸盐(EMIMAc),对5-羟甲基糠醛(HMF)执行安息香缩合以生成5,5' -二(羟甲基)furoin(DHMF)。23由于离子液体可以买到,报价产生NHC的的一种廉价的方式,我们有兴趣在研究什么其他类型的反应,离子液体可以执行。为此,我们发现,二烷基咪唑鎓可有效用作在正规共轭additi预催化剂上的不饱和醛以查耳酮(图1),得到1,6-酮酯。最有效的IL,EMIMAc,促进肉桂醛和查尔之间的高立体选择性反应。发生反应以高偏爱反 -非对映和1,6-酮酯可以分离在产率高达92%。24,25,26
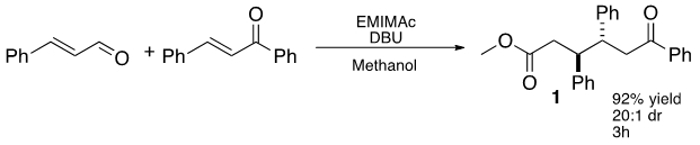
图1:IL-介导的三组分,立体选择性加成肉桂醛以查耳酮。
Protocol
Representative Results
Discussion
基于由酮酯3的X射线分析和对所提出的Bode和同事30的机械调查确定反 -构型的下述反应路径建议(图5)。白细胞介素脱质子产生NHC种;所述NHC反应与不饱和醛形成布瑞斯罗夫中间体I中布瑞斯罗夫中间和在跨安息香反应的查耳酮反应形成二烯二。中级II经过转乘船过渡态(TS)的氧Cope重排,设置防取向的手性中心III。互变异构之后,酰基氮鎓IV与甲醇反应来交付产品?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
We gratefully acknowledge the Swedish Research Council Formas for generous financial support.
Materials
| 1-ethyl-3-methyl imidazolium acetate | Aldrich | 51053-100G-F | Produced by BASF ≥90%, dried on a rotary evaporated before use (10 mBar, 40 °C, 1h) CAS NUMBER: 143314-17-4 |
| 1,3-diphenyl-2-propen-1-one | Aldrich | 11970-100G | 98.0% CAS NUMBER: 94-41-7 |
| trans-cinnamaldehyde | Aldrich | C80687-25G | 99%, stored under nitrogen prior to use CAS NUMBER: 14371-10-9 |
| 1,8-Diazobicyclo[5.4.0]undec-7-ene | Aldrich | 139009-25G | 98% CAS NUMBER: 6674-22-2 |
| Methanol | Sigma-Aldrich | 32213N-2.5L | puriss. P.a., ACS reagent, reag. ISO, reag. Ph. Eur. ≥99.8% (GC) CAS NUMBER: 67-56-1 |
| Dichloromethane | Fischer Chemical | D/1852/17X | Analytic reagent grade, stabilized with amylene CAS NUMBER:9/2/1975 |
| n-Heptane | Fischer Chemical | H/0160/17X | Analytic reagent grade CAS NUMBER: 142-82-5 |
References
- Hallett, J. P., Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 111, 3508-3576 (2011).
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 99, 2071-2084 (1999).
- Vora, H. U., Wheeler, P., Rovis, T. Exploiting acyl and enol azolium intermediates via N-hetero- cyclic carbene-catalyzed reactions of α-reducible aldehydes. Adv. Synth. Catal. 354, 1617-1639 (2012).
- Holloczki, O., et al. Carbenes in ionic liquids. New J. Chem. 34, 3004-3009 (2010).
- Enders, D., Balensiefer, T. Nucleophilic Carbenes in Asymmetric Organocatalysis. Acc. Chem. Res. 37, 534-541 (2004).
- Enders, D., Niemeier, O., Henseler, A. Organocatalysis by N-Heterocyclic Carbenes. Chem. Rev. 107, 5606-5655 (2007).
- List, B. Enamine Catalysis Is a Powerful Strategy for the Catalytic Generation and Use of Carbanion Equivalents. Acc. Chem. Res. 37, 548-557 (2004).
- Nair, V., Bindu, S., Sreekumar, V. N-Heterocyclic carbenes: Reagents, not just ligands!. Angew. Chem. Int. Ed. 43, 5130-5135 (2004).
- Marion, N., Dìez-González, S., Nolan, S. P. N-Heterocyclic Carbenes as Organocatalysts. Angew. Chem. Int. Ed. 46, 2988-3000 (2007).
- Biju, A. T., Kuhl, N., Glorius, F. Extending NHC-Catalysis: Coupling Aldehydes with Unconventional Reaction Partners. Acc. Chem. Res. 44, 1182-1195 (2011).
- Bugaut, X., Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 41, 3511-3522 (2012).
- Davis, h. j., Forrester, K. J. Thiazolium-ion based organic ionic liquids (OILs).1,2 Novel OILs which promote the benzoin condensation. Tetrahedron Lett. 40, 1621-1622 (1999).
- Xu, L. -. W., Gao, Y., Yin, J. -. J., Li, L., Xia, C. -. G. Efficient and mild benzoin condensation reaction catalyzed by simple 1-N-alkyl-3-methylimidazolium salts. Tetrahedron Lett. 46, 5317-5320 (2005).
- Jiang, F. S., Yu, H., Gao, G., Xie, R. G. Benzoin condensation in imidazolium based room-temperature ionic liquids. Chin. Chem. Lett. 16, 321-324 (2005).
- Estager, J., Lévêque, J. M., Turgis, R., Draye, M. Solventless and swift benzoin condensation catalyzed by 1-alkyl-3-methylimidazolium ionic liquids under microwave irradiation. J. Mol. Catal. A: Chem. 256, 261-264 (2006).
- Estager, J., Lévêque, J. -. M., Turgis, R., Draye, M. Neat benzoin condensation in recyclable room-temperature ionic liquids under ultrasonic activation. Tetrahedron Lett. 48, 755-759 (2007).
- Orsini, M., Chiarotto, I., Elinson, M. N., Sotgiu, G., Inesi, A. Benzoin condensation in 1,3-dialkylimidazolium ionic liquids via electrochemical generation of N-heterocyclic carbene. Electrochem. Commun. 11, 1013-1017 (2009).
- Dunn, M. H., Cole, M. L., Harper, J. B. Effects of an ionic liquid solvent on the synthesis of [gamma]-butyrolactones by conjugate addition using NHC organocatalysts. RSC Advances. 2, 10160-10162 (2012).
- Kelemen, Z., Holloczki, O., Nagy, J., Nyulaszi, L. An organocatalytic ionic liquid. Org. Biomol. Chem. 9, 5362-5364 (2011).
- Yu, F. -. L., Zhang, R. -. L., Xie, C. -. X., Yu, S. -. T. Synthesis of thermoregulated phase-separable triazolium ionic liquids catalysts and application for Stetter reaction. Tetrahedron. 66, 9145-9150 (2010).
- Aupoix, A., Vo-Thanh, G. Solvent-free synthesis of alkylthiazolium-based ionic liquids and their use as catalysts in the intramolecular Stetter reaction. Synlett. , 1915-1920 (2009).
- Yu, F. -. L., Jiang, J. -. J., Zhao, D. -. M., Xie, C. -. X., Yu, S. -. T. Imidazolium chiral ionic liquid derived carbene-catalyzed conjugate umpolung for synthesis of [gamma]-butyrolactones. RSC Advances. 3, 3996-4000 (2013).
- Liu, D., Zhang, Y., Chen, E. Y. X. Organocatalytic upgrading of the key biorefining building block by a catalytic ionic liquid and N-heterocyclic carbenes. Green Chem. 14, 2738-2746 (2012).
- Ta, L., Axelsson, A., Bijl, J., Haukka, M., Sundén, H. Ionic Liquids as Precatalysts in the Highly Stereoselective Conjugate Addition of α,β-Unsaturated Aldehydes to Chalcones. Chem. Eur. J. 20, 13889-13893 (2014).
- Nair, V., et al. Nucleophilic Heterocyclic Carbene Catalyzed Annulation of Enals to Chalcones in Methanol: A Stereoselective Synthesis of Highly Functionalized Cyclopentanes. Org. Lett. 11, 2507-2510 (2009).
- Ma, J., Huang, Y., Chen, R. N-Heterocyclic carbene-catalyzed (NHC) three-component domino reactions: highly stereoselective synthesis of functionalized acyclic ϵ-ketoesters. Org. Biomol. Chem. 9, 1791-1798 (2011).
- Domingo, L. R., Saez, J. A., Arno, M. A DFT study on the NHC catalysed Michael addition of enols to α,β-unsaturated acyl-azoliums. A base catalysed C-C bond-formation step. Org. Biomol. Chem. 12, 895-904 (2014).
- Kaeobamrung, J., Mahatthananchai, J., Zheng, P., Bode, J. W. An Enantioselective Claisen Rearrangement Catalyzed by N-Heterocyclic Carbenes. J. Am. Chem. Soc. 132, 8810-8812 (2010).
- Zweep, N., van Esch, J. H. . Functional Molecular Gels. , 1-29 (2014).
- Chiang, P. -. C., Kaeobamrung, J., Bode, J. W. Enantioselective, Cyclopentene-Forming Annulations via NHC-Catalyzed Benzoin−Oxy-Cope Reactions. J. Am. Chem. Soc. 129, 3520-3521 (2007).

