利用停流和小角中子散射测量纳米材料的时间演化
Summary
该协议介绍了使用停止流动的样品环境在小角中子散射测量期间原 位 快速混合多种液体溶液,并研究纳米长度尺度和第二时间尺度上的动力学过程。
Abstract
本文介绍了使用停止流动小角中子散射(SANS)样品环境快速混合液体样品,并在几秒钟到几分钟的时间尺度上研究纳米级动力学过程。停止流动的样品环境使用市售的注射泵混合所需体积的液体样品,然后在大约 1 秒内通过动态混合器注入石英玻璃池。时间分辨SANS数据采集与样品混合同步,以跟踪混合后溶液中纳米结构的演变。
为了最有效地利用中子束时间,我们使用一系列流量选择阀在测量之间自动加载、冲洗和干燥池,从而允许在多次样品进样过程中重复收集数据。重复进样,直到收集到足够的中子散射统计数据。混合设置可以编程为系统地改变条件,以测量不同混合比、样品浓度、添加剂浓度和温度下的动力学。每次进样所需的最小样品量约为 150 μL,具体取决于石英池的光程长度。
在添加剂环糊精存在下,给出了使用这种停止流动样品环境的代表性结果,用于快速脂质交换动力学。囊泡在几秒钟内交换外小叶(外部)脂质,并在数小时内完全交换内部和外部脂质。测量脂质交换动力学需要 原位 混合,以捕获更快(秒)和更慢(分钟)的过程并提取动力学速率常数。相同的样品环境也可用于探测其他类型的液体样品中的分子交换,例如脂质纳米颗粒、蛋白质、表面活性剂、聚合物、乳液或无机纳米颗粒。测量纳米级结构转变和交换或反应系统的动力学将为纳米尺度上演变的过程提供新的见解。
Introduction
小角中子散射 (SANS) 提供了一种独特的方法来测量各种材料的尺寸、形状、相互作用和组织,长度尺度从 ≈1 nm 到 ≈100 nm1,2,3。最近的仪器,包括带有聚焦镜的VSANS(甚小角中子散射)仪器,将极限推向了测量更大长度尺度的极限,最高可达≈1000 nm4,5。通常,中子散射方法固有的独特散射对比在测量纳米级结构的时间演变方面具有几个优势,例如药物制剂中组分的聚集6,聚合物系统中的交联和凝胶反应7,8,膜蛋白的介观结晶9,10,蛋白质的降解和展开11,12,以及二氧化硅基材料的生长13,14,15。独特的散射对比度使时间分辨 SANS (TR-SANS) 成为其他基于停止流量的测量的有用补充。
停止流动混合方法通常在小角X射线散射(SAXS)16,17,18,19,20,21,荧光光谱22,23,24,25,26和光散射27,28,29,30中实现。 31,32 在毫秒时间尺度上研究动力学过程的实验。SANS和SAXS之间的重要区别在于,中子散射是一种无损表征技术,因此,SANS可用于测量同一样品数小时甚至数天,而不会对样品造成电离辐射损伤,这可能发生在高通量的X射线散射实验中33。由于重复的SANS测量不会改变探针分子或样品的化学结构,因此可以在没有光漂白影响的情况下研究时间演变,例如,这可能会使依赖于荧光23,24的动力学测量复杂化。此外,SANS可用于测量高浓度和光学不透明的样品,这些样品通常难以用动态光散射等基于光的技术进行表征。
除了提供纳米尺度的结构信息外,SANS还可用于通过中子散射长度密度对比度的变化来探测这些结构的局部组成。不同元素的散射长度密度(SLD)在元素周期表中随机变化,并且随同一元素的不同同位素而变化。一个常用的例子是氢(1H或H)和氘(2H或D),它们的中子散射长度差异很大。因此,富氢材料,如表面活性剂、脂质、蛋白质、RNA、DNA和其他聚合物,可以使用SANS与氘代溶剂区分开来,而不会显著改变系统的物理性质。然而,需要注意的是,H/D 交换会影响样品中的密度、氢键和相变温度。然而,SANS对富氢材料的独特灵敏度在软物质研究中特别有用,其中感兴趣的样品在基于X射线的技术(如SAXS)中具有较低的散射对比度和信号。同位素取代还使SANS成为通过简单地混合H标记和D标记分子来研究富氢材料中分子交换动力学的有力工具。同位素取代在体积较大的荧光染料大于感兴趣的表面活性剂或脂质分子并且会影响交换动力学的系统中特别有用34,35。
时间分辨 SANS 测量是有利的,因为测量的强度是时间、长度尺度和 SLD 对比度的函数。因此,可以设计TR-SANS实验来探测样品空间分布和组成的时间变化。SANS的这些独特优势为许多软材料系统中的动力学过程提供了重要的见解,例如表面活性剂36,37,38,乳液39,40,41,脂质34,42,43,44,45,46,47,48,49,50和聚合物51,52,53,54,55,56,57,58,59,60,61,62。大多数TR-SANS研究都集中在几分钟到几小时的时间尺度上。然而,许多感兴趣的动力学过程发生在第二时间尺度上,对于理解潜在的机制至关重要。捕获这些早期时间点需要快速混合并原位测量溶液,其中混合与停止流光散射 27、28、29、30、31、32、荧光22、23、24、25、26 和 X 射线期间的数据收集同步16,17,18,19,20,21次实验。这项工作描述了样品环境的使用,该环境旨在快速混合多个液体样品并将混合物注入石英玻璃池中进行TR-SANS测量。混合装置是最近开发的毛细管流变SANS装置63的改编,使用多个注射泵和阀门来控制样品混合并自动清洁细胞。通过将注射泵连接到一系列流量选择阀,可以重复混合、测量、冲洗和干燥多个入口流,以促进秒级时间尺度上的 TR-SANS 测量。
目前的程序假定感兴趣的样品已经确定和制备。我们专注于原位混合设置和收集TR-SANS数据的方法。中子散射数据是在NIST中子研究中心(NCNR)的VSANS仪器上收集的;但是,该程序应适用于其他 SANS 仪器。有兴趣在其他SANS仪器上实施类似协议的读者应咨询当地仪器科学家,以确定最佳仪器配置,以在与感兴趣的动力学过程最相关的所需长度尺度和时间尺度上最大化中子通量。这里提供的数据是使用VSANS上的高通量“白束”配置收集的,以在失去空间分辨率5的情况下最大化中子数。探测器架的位置覆盖一系列散射矢量(q),0.005 Å-1< q<0.5 Å-1,对应于≈130 nm至≈13 nm的长度尺度。散射矢量定义为q = 4 π/λ sin (θ/2),其中λ是中子波长,θ是散射角。
用于TR-SANS测量的停止流动混合装置由多个泵、冲洗注射器、样品注射器、流量选择器以及动态混合器、样品池和混合样品容器组成,如图 1所示。所有密封的流体管路都位于空调外壳内,其中包括注射器、阀门、连接管、动态混合器和样品池。可编程热电空调用于在±1°C内控制10°C至50°C范围内的外壳温度。 请注意,一些外壳绝缘层已被移除,以显示设备的工作部件。主混合装置外壳位于NCNR的NG3 VSANS光束线上的平移台上。使用平移台调整外壳位置,将样品池定位在中子束的路径中(黄色虚线)。
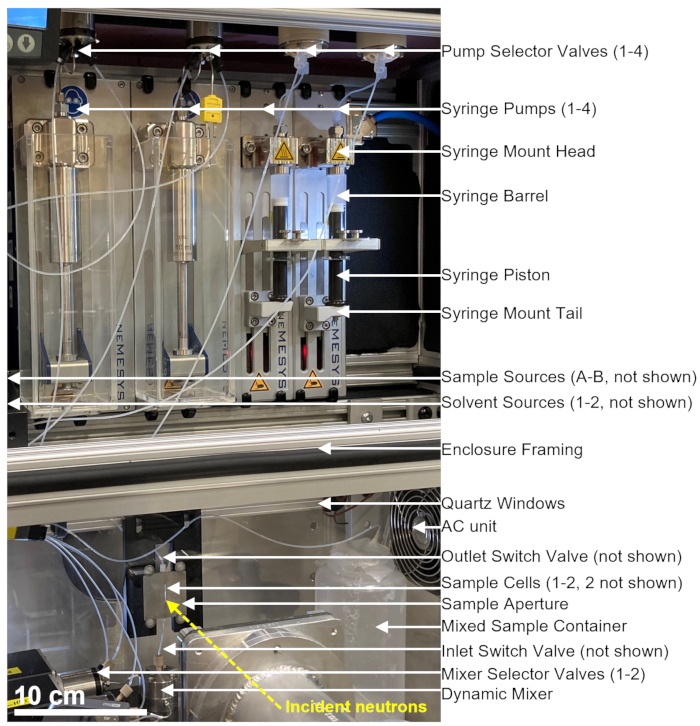
图 1:在 NIST 中子研究中心的 VSANS 光束线上结合停止流混合和小角中子散射测量的示例设置。 该装置包含四个注射泵、两个用于溶剂冲洗的注射器和两个用于样品进样的注射器、四个泵选择阀、两个混合器选择阀、一个动态混合器、一个流通式石英池和一个混合样品容器。入射中子从位于样品池内的混合样品中散射。带有石英窗的绝缘外壳和热电空调装置用于在恒温下控制样品和所有设备。黄色虚线表示中子束路径。比例尺 = 10 厘米。 请点击此处查看此图的大图。
图1所示的设备配置有两个样品注射器、两个冲洗注射器和一个样品池。协议不同步骤的相应流程图如图2所示。将所需体积的两种不同样品注入混合器和样品池(图2A)。样品池填充后,入口开关阀(ISV)和出口开关阀(OSV)关闭,以将样品池与动态混合器隔离,并防止样品在TR-SANS数据收集期间回扩散到池中(图2B)。在动态混合器之前,连接管的长度从10厘米到1米不等,不会影响混合延迟时间。但是,动态混合器和样品池之间的管道连接会影响混合延迟时间和所需的样品进样量。内径为 0.04 英寸(1 毫米)、长度为 100 毫米的预切不锈钢管用于连接动态混合器、混合器选择阀(MSV1 和 MSV2)以及 ISV 和 OSV。内径为 1 mm、长度为 115 mm 的氟化管用于将 ISV 和 OSV(或动态混合器出口)连接到样品池。影响混合延迟时间的总空隙体积包括混合器空隙体积 (0.15 mL)、混合器出口和样品池入口之间的管道 (0.09 mL) 以及样品池体积 (0.16 mL)。在本例中,总空隙体积为 0.4 mL。与管道、混合器和样品池的空隙体积相比,阀门的内部空隙体积可以忽略不计。例如,采用的低压选择阀(0.75 mm 孔径)包含大约 4 μL 的空隙体积,而高压选择阀和开关阀(0.25 mm 孔径)包含大约 0.5 μL 的空隙体积。
TR-SANS测量完成后,用溶剂将样品推出池外,并反复将冲洗溶剂泵入池中以除去残留样品并清洁样品池(图2C)。请注意,冲洗注射器通过泵选择器值连接到较大的溶剂储液槽(例如,水和乙醇),以确保在测量运行之间有足够的溶剂量来清洁样品池。溶剂源、样品源和含有易燃液体的混合样品容器放置在单独的外壳中,没有电气设备,以消除所有可能的点火源。此外,蒸汽锁定瓶盖用于最大限度地减少易燃蒸气和溶剂蒸发。最后,用氮气流干燥样品池以除去残留的冲洗溶剂(图2D)。使用位于氮气瓶上的手动压力调节器,将混合器选择阀的入口氮气压力调节到大约 2 bar(0.2 MPa,表压)。一旦样品池充分清洁和干燥,将新混合的样品注入样品池中以进行下一个测量周期(重复 图2A流程图所示的混合和进样)。
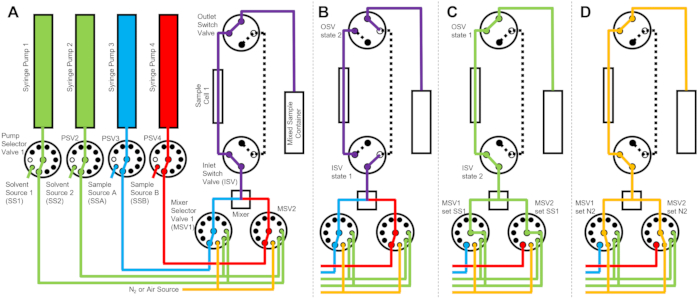
图 2:使用一个样品池、两个样品混合和两种冲洗溶剂进行清洁的示例流程图。 (A)混合样品A(蓝色)和样品B(红色),然后将混合的样品(紫色)流入样品池中。(B)在数据收集期间,ISV和OSV开关阀关闭以隔离样品池并防止样品在数据收集过程中反向扩散的停止流设备状态。(C)数据收集后用SS1(绿色)的漂洗溶剂冲洗样品池的清洁步骤。(D)干燥步骤,其中样品池用氮气(橙色)干燥。缩写:PSV = 泵选择阀;MSV = 混合器选择阀;OSV = 出口开关阀;ISV = 入口开关阀;SS1 = 溶剂源 1;SSA = 样品源 A;N2 = 氮气源。请点击此处查看此图的大图。
图 3 显示了略有不同的版本的流程图,其中混合设置配置为连接到同一开关阀的两个独立样品池(图 3A)。在样品池1中收集TR-SANS数据的同时,对样品池2进行冲洗(图3B)并干燥(图3C)。当样品池 1 的数据收集完成后,入口开关阀将新混合的样品引导到样品池 2 中进行数据收集(图 3D)。在样品池2中收集TR-SANS数据的同时,对样品池1进行冲洗和干燥(图3E)。两个样品池之间的这种交替平行过程最大限度地减少了后续样品进样之间的时间,并最大限度地利用了中子束时间。
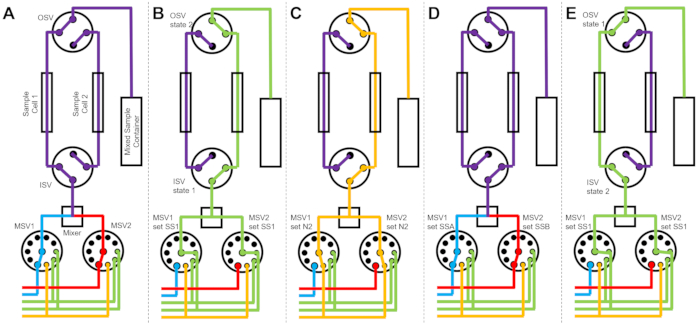
图 3:使用两个样品池、两个样品混合和两个冲洗溶剂进行清洁的示例流程图。 (A)混合样品A(蓝色)和样品B(红色),然后将混合样品(紫色)流入样品池1。(B) 样品池 1 上数据收集期间的停止流动设备状态,同时用 SS1(绿色)中的溶剂冲洗样品池 2。(C) 样品池 1 上数据收集期间的停止流动设备状态,同时样品池 2 用氮气(橙色)干燥。(D)样品池1的数据收集完成后,立即混合新样品(紫色)并流入样品池2。(E) 样品池 2 上数据收集期间的停止流动设备状态,同时用 SS1 中的溶剂冲洗样品池 1(绿色)。在测量一个样品池的同时,正在清洁和干燥另一个样品池。停止流量测量过程在两个样品池之间交替进行,以最大限度地缩短后续样品混合进样之间的时间。缩写:PSV = 泵选择阀;MSV = 混合器选择阀;OSV = 出口开关阀;ISV = 入口开关阀;SS1 = 溶剂源 1;SSA = 样品源 A;N2 = 氮气源。请点击此处查看此图的大图。
下面描述了用于连接泵和管路、启动系统、冲洗和干燥样品池以及注入混合样品的分步方案。虽然为了简单起见,演示了单细胞配置(图2),但可以轻松修改灵活的模块化设置、协议和脚本,以实现更多的注射泵、阀门、混合器或样品池配置,例如图3所示的双样品池配置。在整个混合和清洗注射周期中收集的代表性原始中子计数速率数据如图4所示,而在3种不同温度下测量的脂质交换动力学和对应于交换脂质分数的提取归一化散射强度分别显示在图5和图6中。
Protocol
Representative Results
Discussion
当前程序描述了混合装置和执行截止流TR-SANS测量的步骤。该装置和方案针对低粘度液体样品进行了优化,其中感兴趣的时间尺度为 ≈1 秒至 5 分钟。对于大于5 min的时间尺度,手动混合样品并将其加载到标准散射池中可能更容易且可取,特别是对于高粘度样品,凝胶或糊剂。访问小于 1 s 的时间尺度需要不同的混合设备、更低的总空隙体积和更高的总流速来降低延迟时间。同样重要的是要注意,?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
NG3 VSANS由高分辨率中子散射中心提供,该中心是美国国家标准与技术研究所与美国国家科学基金会之间的合作伙伴关系,协议编号为DMR-2010792。M.H.L.N 感谢 Mitacs Globalink(加拿大)提供的资金。识别任何商业产品或商品名称是为了促进理解,并不意味着国家标准与技术研究院的认可或推荐。
Materials
| Dynamic mixer | Analytical Scientific Instruments | 462-0150A | Magnetically coupled rotor, binary dynamic mixer assembly (ternary type available), 0.15 mL dead volume (larger dead volume available) |
| Fluoropolymer tubing | IDEX Health & Science | 1507L | PFA Tubing Natural 1/16 inch OD x 0.040 inch ID x 50 ft |
| Fluoropolymer 1/4-28 flangeless fittings | IDEX Health & Science | XP-245 | PFA flangeless fitting with ferrules, 1/4-28 threading, 1/16 inch OD tubing |
| Glass syringes | Hamilton Company | 81660 | Hamilton 1000 series syringes, 10 mL (81660), model 1010 C syr, 1/4"-28 thread termination, other volumes available |
| High-pressure flow selector valves | Vici Valco | C85X-1570EUTB | Vici 10 position selector valves, 15000 psi max, 0.25 mm bore, 1/16 inch OD tubing, 10-32 coned threaded ports, USB universal actuator |
| High-pressure switch valves | Vici Valco | C82X-1574EUHB | Vici 4 port switch valves, 15000 psi max, 0.25 mm bore, 1/16 inch OD tubing, 10-32 coned threaded ports, USB universal actuator |
| High-pressure syringes | Cetoni | A2019000358 | 3 mL stainless steel syringe, 510 bar max, 21 mL/min flow rate max |
| Low-pressure flow selector valves | Vici Valco | C25-3180EUHB | Vici 10 position selector valves, max 250 psi liquid, 0.75 mm bore, 1/16 inch OD tubing, 1/4-28 threaded ports, USB universal actuator |
| neMESYS high-pressure syringe pumps | Cetoni | A3921000103 | Max force 2600 N |
| neMESYS mid-pressure syringe pumps | Cetoni | A3921000131 | Max force 1000 N |
| Power supply | Cetoni | A3921000127 | Base 600, supplies power for up to 4 high pressure pumps |
| Quartz flow-through sample cell | Starna Scientific | 3-2.30-Q-1/TC | Quartz micro flow cells, 2 mm path length (1 mm available), 2 mm by 2 mm by 30 mm internal dimension |
| Quartz windows | Technical Glass Products | NA | GE 124 Clear fused quartz ground and polished plates, 11.75 inch by 23.75 inch by 0.375 inch thick |
| Stainless steel 10-32 coned compression fittings | IDEX Health & Science | U-321X, U-320X | 316 stainless steel ferrule (U-321X) and nut (U-320X) -Valco type, 10-32 coned, for 1/16 inch OD stainless steel tubing |
| Stainless steel tubing | IDEX Health & Science | U-102 | Stainless Steel Tubing 1/16 inch OD x 0.020 inch ID, 10 cm, various precut lengths available |
| Syringe pump control software | Cetoni | T6000000004 | QmixElements software for nemesys pumps, QmixSDK software development kit |
| Thermoelectric air conditioner | EIC Solutions | AAC-140C-4XT-HC | Thermoelectric air conditioner mounted on insulated enclosure to control the pump, valve, mixer, and sample temperature |
| T-slot railing | McMaster-Carr | 47065T103 | Aluminum t-slotted railing (1.5 inch by 1.5 inch) cut to various lengths |
| Vapor locking bottle caps | Cole-Parmer | EW-12018-02 | Four 304 SS port inserts, 1/4"-28 threads, GL45 bottle cap size, PTFE body, SS threads, PP collar |
References
- Melnichenko, Y. B., Wignall, G. D. Small-angle neutron scattering in materials science: Recent practical applications. Journal of Applied Physics. 102 (2), 021101 (2007).
- Grillo, I., Borsali, R., Pecora, R. Small-angle neutron scattering and applications in soft condensed matter. Soft Matter Characterization. , (2008).
- Hollamby, M. J. Practical applications of small-angle neutron scattering. Physical Chemistry Chemical Physics. 15 (26), 10566-10579 (2013).
- Pipich, V., Fu, Z. KWS-3: Very small angle diffractor with focusing mirror. Journal of large-scale research. 1, 31 (2015).
- Barker, J. G., Kline, S., et al. . 2019 NCNR Annual Report, Special Publication (NIST SP). , (2019).
- Gilbert, P. H., et al. Preservative induced polysorbate 80 micelle aggregation. Journal of Pharmaceutical Sciences. 10 (6), 2395-2404 (2021).
- Terashima, T., et al. In situ and time-resolved small-angle neutron scattering observation of star polymer formation via arm-linking reaction in ruthenium-catalyzed living radical polymerization. Macromolecules. 43 (19), 8218-8232 (2010).
- Hashimoto, K., Fujii, K., Nishi, K., Shibayama, M. Ion gel network formation in an ionic liquid studied by time-resolved small-angle neutron scattering. The Journal of Physical Chemistry B. 122 (40), 9419-9424 (2018).
- Conn, C. E., et al. Membrane protein structures in lipid bilayers; small-Angle neutron scattering with contrast-matched bicontinuous cubic phases. Frontiers in Chemistry. 8, 619470 (2021).
- van’t Hag, L., et al. Protein-eye view of the in meso crystallization mechanism. Langmuir. 35 (25), 8344-8356 (2019).
- Mahieu, E., et al. Observing protein degradation by the PAN-20S proteasome by time-resolved neutron scattering. Biophysical Journal. 119 (2), 375-388 (2020).
- Ibrahim, Z., et al. Time-resolved neutron scattering provides new insight into protein substrate processing by a AAA+ unfoldase. Scientific Reports. 7 (1), 40948 (2017).
- Hollamby, M. J., et al. Growth of mesoporous silica nanoparticles monitored by time-resolved small-angle neutron scattering. Langmuir. 28 (9), 4425-4433 (2012).
- Blin, J. L., Impéror-Clerc, M. Mechanism of self-assembly in the synthesis of silica mesoporous materials: in situ studies by X-ray and neutron scattering. Chemical Society Reviews. 42 (9), 4071-4082 (2013).
- Impéror-Clerc, M., Grillo, I., Khodakov, A. Y., Durand, D., Zholobenko, V. L. New insights into the initial steps of the formation of SBA-15 materials: an in situ small angle neutron scattering investigation. Chemical Communications. 8, 834-836 (2007).
- Narayanan, T., Rüter, A., Olsson, U. SAXS/WAXS investigation of amyloid-β(16-22) peptide nanotubes. Frontiers in Bioengineering and Biotechnology. 9, 654349 (2021).
- Angelov, B., et al. DNA/Fusogenic lipid nanocarrier assembly: millisecond structural dynamics. The Journal of Physical Chemistry Letters. 4 (11), 1959-1964 (2013).
- Amann, M., et al. Kinetic pathways for polyelectrolyte coacervate micelle formation revealed by time-resolved synchrotron SAXS. Macromolecules. 52 (21), 8227 (2019).
- Varga, Z., Wacha, A., Bóta, A. Osmotic shrinkage of sterically stabilized liposomes as revealed by time-resolved small-angle X-ray scattering. Journal of Applied Crystallography. 47 (1), 35-40 (2014).
- Panine, P., Finet, S., Weiss, T. M., Narayanan, T. Probing fast kinetics in complex fluids by combined rapid mixing and small-angle X-ray scattering. Advances in Colloid and Interface Science. 127 (1), 9-18 (2006).
- Grillo, I. Applications of stopped-flow in SAXS and SANS. Current Opinion in Colloid & Interface Science. 14 (6), 402-408 (2009).
- Gomez-Hens, A., Perez-Bendito, D. The stopped-flow technique in analytical chemistry. Analytica Chimica Acta. 242, 147-177 (1991).
- Patel, J. T., Belsham, H. R., Rathbone, A. J., Friel, C. T. Use of stopped-flow fluorescence and labeled nucleotides to analyze the ATP turnover cycle of kinesins. Journal of Visualized Experiments: JoVE. (92), e52142 (2014).
- Biro, F. N., Zhai, J., Doucette, C. W., Hingorani, M. M. Application of stopped-flow kinetics methods to investigate the mechanism of action of a DNA repair protein. Journal of Visualized Experiments: JoVE. (37), e1874 (2010).
- Raney, K. D., Sowers, L. C., Millar, D. P., Benkovic, S. J. A fluorescence-based assay for monitoring helicase activity. Proceedings of the National Academy of Sciences of the United States of America. 91 (14), 6644-6648 (1994).
- Roder, H., Maki, K., Cheng, H. Early events in protein folding explored by rapid mixing methods. Chemical reviews. 106 (5), 1836-1861 (2006).
- Milon, A., et al. Osmotic swelling of unilamellar vesicles by the stopped-flow light scattering method. Influence of vesicle size, solute, temperature, cholesterol and three α,ω-dihydroxycarotenoids. Biochimica et Biophysica Acta (BBA) – Biomembranes. 859 (1), 1-9 (1986).
- Gast, K., Nöppert, A., Müller-Frohne, M., Zirwer, D., Damaschun, G. Stopped-flow dynamic light scattering as a method to monitor compaction during protein folding. European Biophysics Journal. 25 (3), 211-219 (1997).
- Antoun, A., Pavlov, M. Y., Tenson, T., Ehrenberg, M. M. Ribosome formation from subunits studied by stopped-flow and Rayleigh light scattering. Biological Procedures Online. 6, 35-54 (2004).
- Zhu, Z., Armes, S. P., Liu, S. pH-Induced micellization kinetics of ABC triblock copolymers measured by stopped-flow light scattering. Macromolecules. 38 (23), 9803-9812 (2005).
- Ye, J., et al. Comparative study of temperature-induced association of cyclic and linear poly(N-isopropylacrylamide) chains in dilute solutions by laser light scattering and stopped-flow temperature jump. Macromolecules. 41 (12), 4416-4422 (2008).
- Liu, X., et al. Early stage kinetics of polyelectrolyte complex coacervation monitored through stopped-flow light scattering. Soft Matter. 12 (44), 9030-9038 (2016).
- Garman, E. F., Weik, M. X-ray radiation damage to biological samples: recent progress. Journal of Synchrotron Radiation. 26 (4), 907-911 (2019).
- Garg, S., Porcar, L., Woodka, A. C., Butler, P. D., Perez-Salas, U. Noninvasive neutron scattering measurements reveal slower cholesterol transport in model lipid membranes. Biophysical Journal. 101 (2), 370-377 (2011).
- Marquardt, D., et al. 1H NMR shows slow phospholipid flip-flop in gel and fluid bilayers. Langmuir. 33 (15), 3731-3741 (2017).
- Egelhaaf, S. U., Olsson, U., Schurtenberger, P. Time-resolved SANS for surfactant phase transitions. Physica B: Condensed Matter. 276-278, 326-329 (2000).
- Tabor, R. F., Eastoe, J., Grillo, I. Time-resolved small-angle neutron scattering as a lamellar phase evolves into a microemulsion. Soft Matter. 5 (10), 2125-2129 (2009).
- Gradzielski, M., Bergmeier, M., Hoffmann, H., Müller, M., Grillo, I. Vesicle gel formed by a self-organization process. The Journal of Physical Chemistry B. 104 (49), 11594-11597 (2000).
- Lee, Y. -. T., Li, D. S., Pozzo, L. D. Kinetic analysis of ultrasound-induced oil exchange in oil-in-water emulsions through contrast variation time-resolved small-sngle neutron scattering. Langmuir. 35 (47), 15204-15213 (2019).
- Lee, Y. -. T., Pozzo, L. D. Contrast-variation time-resolved small-angle neutron scattering analysis of oil-exchange kinetics between oil-in-water emulsions stabilized by anionic surfactants. Langmuir. 35 (47), 15192-15203 (2019).
- Roger, K., Olsson, U., Schweins, R., Cabane, B. Emulsion ripening through molecular exchange at droplet contacts. Angewandte Chemie International Edition. 54 (5), 1452-1455 (2015).
- Nakano, M., Fukuda, M., Kudo, T., Endo, H., Handa, T. Determination of Interbilayer and Transbilayer Lipid Transfers by Time-Resolved Small-Angle Neutron Scattering. Physical Review Letters. 98 (23), 238101 (2007).
- Nakano, M., et al. Flip-flop of phospholipids in vesicles: kinetic analysis with time-resolved small-angle neutron scattering. The Journal of Physical Chemistry B. 113 (19), 6745-6748 (2009).
- Nguyen, M. H. L., et al. Methanol accelerates DMPC flip-flop and transfer: A SANS study on lipid dynamics. Biophysical Journal. 116 (5), 755-759 (2019).
- Nguyen, M. H. L., et al. Peptide-induced Lipid flip-flop in asymmetric liposomes measured by small angle neutron scattering. Langmuir. 35 (36), 11735-11744 (2019).
- Nguyen, M. H. L., et al. Time-resolved SANS reveals pore-forming peptides cause rapid lipid reorganization. New Journal of Chemistry. 45 (1), 447-456 (2021).
- Xia, Y., et al. Effects of nanoparticle morphology and acyl chain length on spontaneous lipid transfer rates. Langmuir. 31 (47), 12920-12928 (2015).
- Xia, Y., et al. Morphology-induced defects enhance lipid transfer rates. Langmuir. 32 (38), 9757-9764 (2016).
- Maric, S., et al. Time-resolved small-angle neutron scattering as a probe for the dynamics of lipid exchange between human lipoproteins and naturally derived membranes. Scientific Reports. 9 (1), 7591 (2019).
- Nielsen, J. E., Bjørnestad, V. A., Pipich, V., Jenssen, H., Lund, R. Beyond structural models for the mode of action: How natural antimicrobial peptides affect lipid transport. Journal of Colloid and Interface Science. 582, 793-802 (2021).
- Willner, L., Poppe, A., Allgaier, J., Mokenbusch, M., Richter, D. TIme-resolved SANS for the determintioan of unimer exchange kinetics in block copolymer micelles. Europhysics Letters. 55 (5), 667 (2001).
- Lund, R., Willner, L., Stellbrink, J., Lindner, P., Richter, D. Logarithmic chain-exchange kinetics of diblock copolymer micelles. Physical Review Letters. 96 (6), 068302 (2006).
- Lund, R., Willner, L., Richter, D., Dormidontova, E. E. Equilibrium chain exchange kinetics of diblock copolymer micelles: Tuning and logarithmic relaxation. Macromolecules. 39 (13), 4566-4575 (2006).
- Lund, R., Willner, L., Richter, D., Abe, A., Lee, K. S., Leibler, L., Kobayashi, S. Kinetics of block copolymer micelles studied by small-angle scattering methods. in Controlled Polymerization and Polymeric Structures. Advances in Polymer Science. , 51 (2013).
- Choi, S. -. H., Lodge, T. P., Bates, F. S. Mechanism of molecular exchange in diblock copolymer micelles: hypersensitivity to core chain length. Physical Review Letters. 104 (4), 047802 (2010).
- Choi, S. -. H., Bates, F. S., Lodge, T. P. Molecular exchange in ordered diblock copolymer micelles. Macromolecules. 44 (9), 3594-3604 (2011).
- Lu, J., Bates, F. S., Lodge, T. P. Chain exchange in binary copolymer micelles at equilibrium: confirmation of the independent chain hypothesis. ACS Macro Letters. 2 (5), 451-455 (2013).
- Lu, J., Bates, F. S., Lodge, T. P. Remarkable effect of molecular architecture on chain exchange in triblock copolymer micelles. Macromolecules. 48 (8), 2667-2676 (2015).
- Kelley, E. G., et al. Size evolution of highly amphiphilic macromolecular solution assemblies via a distinct bimodal pathway. Nature Communications. 5 (1), 3599 (2014).
- Murphy, R. P., Kelley, E. G., Rogers, S. A., Sullivan, M. O., Epps, T. H. Unlocking chain exchange in highly amphiphilic block polymer micellar systems: influence of agitation. ACS Macro Letters. 3 (11), 1106-1111 (2014).
- Schantz, A. B., et al. PEE-PEO block copolymer exchange rate between mixed micelles is detergent and temperature activated. Macromolecules. 50 (6), 2484-2494 (2017).
- Lantz, K. A., et al. Cavitation enables switchable and rapid block polymer exchange under high-χN conditions. Macromolecules. 51 (17), 6967-6975 (2018).
- Murphy, R. P., et al. Capillary RheoSANS: measuring the rheology and nanostructure of complex fluids at high shear rates. Soft Matter. 16 (27), 6285-6293 (2020).
- Stopped Flow Sans. usnistgov Available from: https://github.com/usnistgov/stopped-flow-sans (2021)
- Kline, S. Reduction and analysis of SANS and USANS data using IGOR Pro. Journal of Applied Crystallography. 39 (6), 895-900 (2006).
- Doktorova, M., et al. Preparation of asymmetric phospholipid vesicles for use as cell membrane models. Nature Protocols. 13 (9), 2086-2101 (2018).
- Huang, Z., London, E. Effect of cyclodextrin and membrane lipid structure upon cyclodextrin-lipid interaction. Langmuir. 29 (47), 14631-14638 (2013).
- Sugiura, T., Ikeda, K., Nakano, M. Kinetic analysis of the methyl-β-cyclodextrin-mediated intervesicular transfer of pyrene-labeled phospholipids. Langmuir. 32 (51), 13697-13705 (2016).
- Scott, H. L., et al. On the mechanism of bilayer separation by extrusion, or why your LUVs are not really unilamellar. Biophysical Journal. 117 (8), 1381-1386 (2019).
- Dicko, C., et al. NUrF-Optimization of in situ UV-vis and fluorescence and autonomous characterization techniques with small-angle neutron scattering instrumentation. Review of Scientific Instruments. 91 (7), 075111 (2020).

