Laser Capture Microdissection of Neurons from Differentiated Human Neuroprogenitor Cells in Culture
Summary
Human neuroprogenitor cells (NPCs) were expanded under proliferating conditions. NPCs were differentiated into neuron-rich cultures in the presence of a combination of neurotrophins. Neuronal markers were detected by immunofluorescence staining. To isolate a pure population of neurons, NPCs were differentiated on PEN membrane slides and laser capture microdissection was performed.
Abstract
Neuroprogenitor cells (NPCs) isolated from the human fetal brain were expanded under proliferative conditions in the presence of epidermal growth factor (EGF) and fibroblast growth factor (FGF) to provide an abundant supply of cells. NPCs were differentiated in the presence of a new combination of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), dibutyryl cAMP (DBC) and retinoic acid on dishes coated with poly-L-lysine and mouse laminin to obtain neuron-rich cultures. NPCs were also differentiated in the absence of neurotrophins, DBC and retinoic acid and in the presence of ciliary neurotrophic factor (CNTF) to yield astrocyte-rich cultures. Differentiated NPCs were characterized by immunofluorescence staining for a panel of neuronal markers including NeuN, synapsin, acetylcholinesterase, synaptophysin and GAP43. Glial fibrillary acidic protein (GFAP) and STAT3, astrocyte markers, were detected in 10-15% of differentiated NPCs. To facilitate cell-type specific molecular characterization, laser capture microdissection was performed to isolate neurons cultured on polyethylene naphthalate (PEN) membrane slides. The methods described in this study provide valuable tools to advance our understanding of the molecular mechanism of neurodegeneration.
Introduction
Life-long neurogenesis is known to occur in the subventricular zone of the lateral ventricles and in the subgranular layer of the dentate gyrus of the adult mammalian brain 1. Neuroprogenitor cells (NPCs) that originate from these regions are multipotent cells that can differentiate into neurons, astrocytes and oligodendrocytes 2. NPCs have generated interest because of their potential to be transplanted in patients with various neurodegenerative disorders including Parkinson’s disease, amyotrophic lateral sclerosis, stroke and Alzheimer’s disease (AD) 3. Studies with NPCs have generally focused on this transplantation angle but the potential of NPC-derived neurons as a cell culture model to determine the mechanism of neurodegeneration has not been fully exploited. Previous studies have generally used post-mitotic neurons isolated from rodent brain tissues which need to be isolated for each experiment as they are not self-renewing. Although human neuroblastoma cell lines including SH-SY5Y and SK-N-MC cells can be expanded, they do not have the characteristics of primary neurons. Human NPCs, on the other hand, offer both advantages because they can be expanded for multiple passages and can be differentiated to generate a cell population with the characteristics of primary neurons 4,5. In the current study, we describe a new differentiation protocol to obtain a consistent neuron-rich population from commercially available NPCs isolated from human fetal brain. Because these cultures do contain a small percent of glial cells, we need additional methods to isolate a pure population of neurons for molecular characterization. Laser capture microdissection (LCM) is a novel technique by which a homogenous population of cells from a tissue section can be selectively captured for gene expression analysis 6. The brain is a heterogeneous tissue consisting of neurons, glia and other cell types. LCM has been used to determine neuron-specific gene expression analyses 7-10. We have previously performed LCM of hippocampal neurons from AD (Tg2576) mouse brain sections to show decreased expression of cyclic AMP response element binding protein (CREB) and BDNF specifically in hippocampal neurons 11. In the present study, we describe procedures for the expansion of human NPCs, neuronal differentiation, immunofluorescent staining for neuronal markers and LCM for the isolation of neurons cultured on PEN membrane slides.
Protocol
1. Expansion of Human NPCs (Figure 1)
- Revive the frozen stock of human NPCs from fetal brain (Lonza, Walkersville, MD, USA) and culture them in suspension in T-75 flasks as neurospheres (Figure 1A) in neurobasal medium containing proliferation supplements, EGF (10 ng/ml) and FGF (10 ng/ml).
- After 3 days in culture, transfer the neurospheres to a 15 ml tube and centrifuge at 500 rpm for 5 min.
- Discard the supernatant leaving behind ~100 μl medium above the cell pellet and transfer into an Eppendorf tube. A 100 μl cell pellet is enough to split into 2 T-75 flasks. If less, reduce the number of flasks as neuroprogenitor cells fail to proliferate if split thin.
- Triturate the pellet with a 200 μl tip 50x while keeping the 200 μl tip against the bottom of the tube. Divide the cell suspension equally into two parts and add them to 2 T-75 flasks (1-2 split), one for continued expansion and another for differentiation.
- Continue the culture of the neuroprogenitor cells for expansion (first flask) by changing the medium every 4-5 days until the neurospheres reach their original size of 300-500 μm. Change medium by centrifugation (500 rpm; 5 min), discard the old medium and add new medium.
2. Differentiation of Human NPCs into a Neuron-rich Culture (Figure 2)
- For differentiation of neuroprogenitor cells into a neuron rich culture, place a single coverslip into each well of a 24-well plate and coat with 100 μg/ml of poly-L-lysine by adding 500 μl into each well. Incubate the dishes for 30 min at RT.
- Aspirate the poly-L-lysine solution and rinse the plate with sterile water.
- Next, coat the wells of the plate with 500 μl of 5 μg/ml mouse laminin and incubate for 30 min. Following incubation, wash the wells with PBS.
- 4 days after splitting the cells (step 1.4), transfer the small neurospheres in the flask labeled 'For differentiation' into the coated dishes at a density of ~500 neurospheres per well of a 24-well plate.
- After 6 hr, when the neurospheres attach to the dish, remove the proliferation medium and add differentiation medium consisting of neurobasal medium, B27 supplement, NGF (20 ng/ml), BDNF (10 ng/ml), DBC (100 mM), and retinoic acid (2 μM).
3. Immunofluorescence Staining of Differentiated NPCs (Figure 3)
- Following culture of neuroprogenitor cells in neuronal differentiation medium for two weeks, a neuron-rich culture is obtained. Rinse the neurons once in PBS and then fix them in 4% paraformaldehyde for 30 min. Once fixed, rinse the cells three times with PBS.
- Incubate the cells with permeabilization buffer (5% BSA and 0.2% Triton X-100 in PBS) at RT for 60 min.
- Incubate the dishes O/N at 4 °C in a shaker with the following combination of polyclonal and monoclonal antibodies in 3% BSA in PBS: NeuN (1:250) and synapsin (1:250); acetyl cholinesterase (1:500) and synaptophysin (1:250); BDNF (1:500) and GAP43 (1:250); STAT3 (1:500) and GFAP (1:1,000).
- Wash the coverslips three times with PBS and then incubate them with anti-rabbit-Cy3 and anti-mouse-FITC secondary antibodies at RT in dark for 90 min. Following incubation, wash the coverslips three times with PBS.
- Place 10 μl of mounting medium onto a glass slide, take out the coverslip from the culture dish with forceps, and place it upside down on the mounting medium. Gently, wipe away any excess mounting medium and seal the edges with nail polish.
- Examine the immunostained neurons in a fluorescence microscope.
4. Laser Capture Microdissection of Neurons (Figure 4)
- Differentiate NPCs into a neuron-rich culture on PEN membrane slides coated with poly-L-lysine and mouse laminin following the procedures described for Figure 2.
- Perform all subsequent steps under RNAse-free conditions.
- Stain the cultures using HistoGene Stain (Arcturus) to visualize the cells.
- Find the areas of pure neuronal populations devoid of astrocytes using the road map image of the entire slide with the Veritas LCM system. Mark the neurons using the drawing tools.
- Perform laser capture of these areas using computer-controlled precision and automation in a two-step process. First, IR (Infrared) is fired at multiple spots with a laser power setting of 70 mW and a pulse of 2,500 μsec to get the membrane attach to the cap. Next, the marked area is excised using an UV cutting tool at a low level setting of 10 mV.
- Combination of these processes selectively captures the marked areas from the PEN membrane onto CapSure LCM macro caps. When the cap is lifted, the membrane with the neurons adheres to the cap.
- Isolate total RNA from LCM samples using a PicoPure RNA isolation kit (Arcturus) and treat with DNase.
- Amplify the isolated RNA using RiboAMP RNA amplification kit following instructions from the kit.
- Perform Real time RT-PCR analysis using TaqMan probes to detect human neurofilament heavy chain (hNFHc).
Abbreviations:
AD, Alzheimer’s disease; BDNF, brain-derived neurotrophic factor; CREB, cyclic AMP response element binding protein; DBC, Dibutyryl cyclic AMP; EGF, epidermal growth factor; FGF, fibroblast growth factor; GFAP, glial fibrillary acidic protein; LCM, laser capture microdissection; NGF, Nerve growth factor; NPC, neuroprogenitor cell; PEN, polyethylene naphthalate.
Representative Results
Expansion of NPCs (Figure 1)
When neurospheres are broken down to a single cell suspension by trituration, the pipette tip needs to touch the bottom of the tube so that there is some resistance when the suspension is pipetted up and down. The number of times of trituration will vary between individuals and needs to be decided by trial and error by examining the resulting cell suspension under a microscope. It is critical to provide sufficient density of cell suspension because the proliferation will be at a faster rate when the NPCs come in close contact. If NPCs do not grow, the cell density should be increased by reducing the number of flasks. We have been able to expand the neurospheres for up to 10-15 passages. Thus abundant supply of human NPCs can be generated, reducing the use of human fetal tissue. For continued supply of NPCs and neurons, it is desirable to expand them initially for 3-4 passages and subsequently, half of the NPCs can be expanded as neurospheres and the other half can be differentiated for ongoing experiments. Alternatively, NPCs can be also expanded for more passages, frozen in liquid nitrogen as in the case of cell lines to generate larger supply of stocks, and revived when needed. It is also desirable to perform experiments with neurons derived from different batches of NPCs.
Neuronal Differentiation of NPCs (Figure 2)
NPCs are multipotent cells and can be differentiated into neurons, astrocytes or oligodendrocytes depending on the culture conditions. Our differentiation protocol involved seeding of 4-day old neurospheres in coated dishes (Figure 2A). We observed that it is essential to seed sufficient density of neurospheres so that the differentiating neurons spread around and form a dense network of neuronal processes. Images after 1 (Figure 2B) and 4 days (Figure 2C) of seeding are shown. Neuron-rich cultures yielding 80-90% neurons and 10-15% astrocytes, were obtained after differentiation of NPCs in the presence of a combination of NGF, BDNF, DBC and retinoic acid for two weeks (Figure 2D). To the best of our knowledge, the protocol described in this study for neuronal differentiation has not been reported previously by others. In areas of low density, NPCs differentiated into astrocytes (Figure 2E). To obtain an astrocyte-rich culture, NPCs can be cultured in the presence of CNTF (10 ng/ml) and in the absence of NGF, BDNF, DBC and retinoic acid. Thus the neuron-astrocyte composition can be manipulated during differentiation depending on the objective of the experiment.
Characterization of Differentiated Neurons (Figure 3)
To further characterize these differentiated NPCs, we performed dual immunostaining for neuronal markers with polyclonal and monoclonal antibodies, followed by exposure to rabbit and mouse secondary antibodies linked to Cy3 (red) and FITC (green) respectively. The following neuronal markers were detected: Figure 3A: NeuN and synapsin. Figure 3B: Acetyl cholinesterase (Ache) and synaptophysin. Figure 3C: BDNF and GAP43. Thus, the differentiated NPCs have the characteristics of primary neurons. In addition, astrocyte markers STAT3 and GFAP were detected in a small population of cells (Figure 3A). Therefore additional methods are needed for neuron-specific gene expression analysis.
Laser Capture Microdissection (LCM) of Neurons (Figure 4)
Although LCM can be used for isolation of cells from a culture dish, our initial attempts of LCM with neurons failed because they are firmly attached to the coated dishes. To overcome this problem, we differentiated NPCs on slides with PEN membrane inserts coated with poly-L-lysine and mouse laminin. The slide was placed inside a 100 mm cell culture dish (Figure 4A). The arrangements of the PEN membrane slide and LCM caps are shown in Figure 4B. Cultures were stained with HistoGen stain (Arcturus) to visualize the cells (Figure 4C). The slide was placed in the microscope in the Arcturus Veritas instrument. The neurons to be captured were marked with drawing tools (Figure 4D). CapSure HS Caps were placed on the PEN membrane. Using a combination of UV laser cutting and IR laser capture, the marked areas were collected onto the cap. The captured neurons are shown on the cap at low (Figure 4F) and high (Figure 4F) magnification.
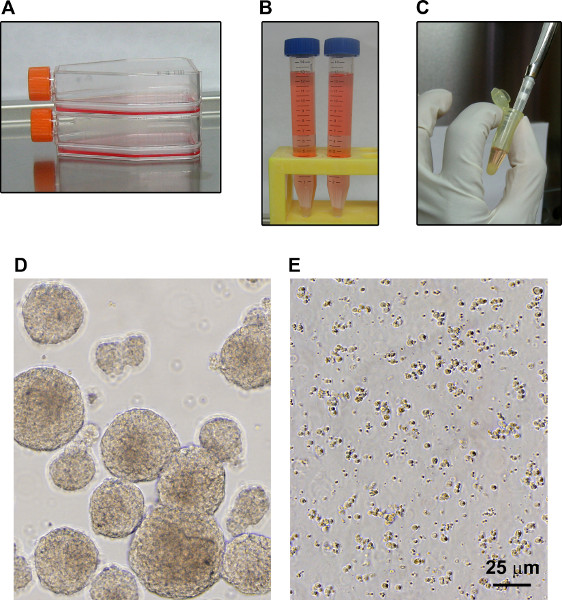
Figure 1. Expansion of human NPCs. A. NPCs were cultured in suspension in T75 flasks as neurospheres. B. When the neurospheres reached the size of 300-500 μm, they were transferred to 15 ml tubes and centrifuged at 1,000 RPM for 5 min. C. The supernatant was discarded and the cell pellet in 200 μl medium was transferred to Eppendorf tubes and triturated. D. Neurospheres ready to be split are shown. E. Broken neurospheres following trituration are shown.
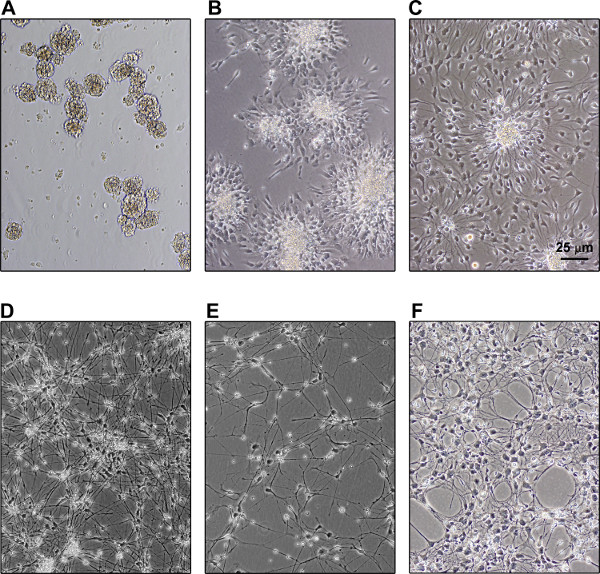
Figure 2. Differentiation of human NPCs. A. Neurospheres were broken by trituration and cultured for four days to generate new neurospheres. B. Four day-old neurospheres were seeded in dishes coated with poly-L-lysine and mouse laminin. C. Differentiation of NPCs was observed in the presence of NGF, BDNF, DBC and retinoic acid in three days after seeding. D. Neuron-rich culture was obtained after two weeks. E. NPCs differentiated into astrocytes in areas of low density. F. NPCs differentiated into an astrocyte-rich culture in the presence of CNTF (10 ng/ml) and in the absence of NGF, BDNF, DBC and retinoic acid.
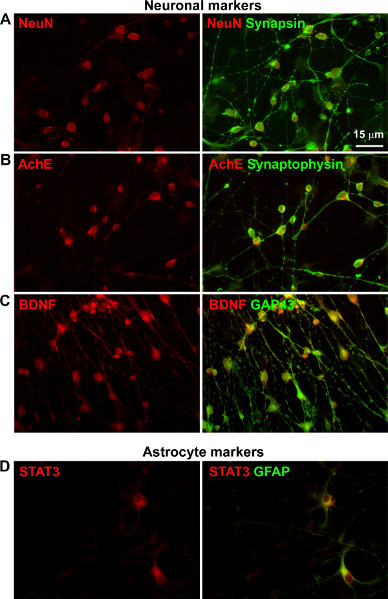
Figure 3. Neuronal and astrocyte markers in differentiated human NPCs. NPCs differentiated for two weeks were fixed and dual immunostained for indicated targets with polyclonal and monoclonal antibodies, followed by exposure to rabbit and mouse secondary antibodies linked to Cy3 (red) and FITC (green) respectively. The following neuronal markers were detected: A. NeuN and synapsin. B. Acetyl cholinesterase (AchE) and synaptophysin. C. BDNF and GAP43. In addition, the following astrocyte markers were detected: D. STAT3 and GFAP.
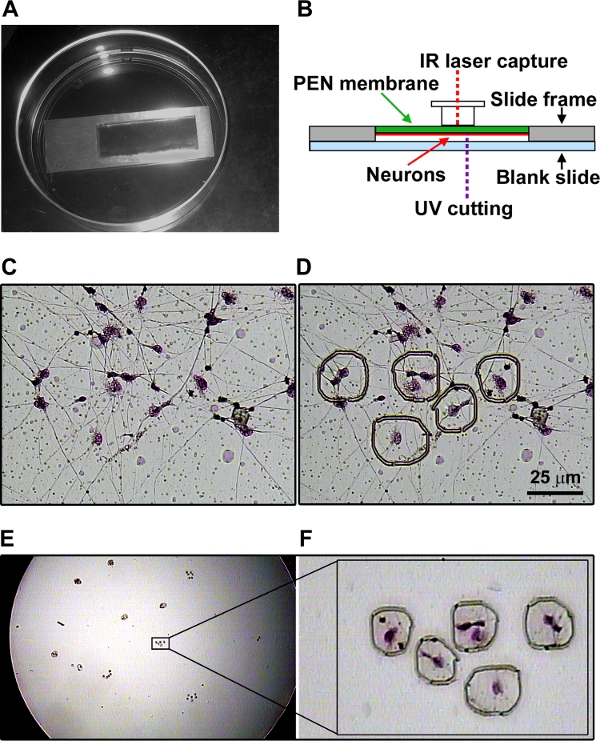
Figure 4. Laser capture microdissection of neurons. A. NPCs were differentiated on a PEN membrane slide coated with poly L lysine and mouse laminin. The slide was placed inside a 100 mm cell culture dish. B. The arrangements of PEN membrane slide and LCM cap in the Arcturus Veritas instrument are shown. C. Cultures were stained with HistoGen stain (Arcturus) to visualize the cells. D. The neurons to be captured were marked with drawing tools. The captured neurons are shown on the cap at low (E) and high (F) magnification. Click here to view larger figure.
Discussion
We describe in this study a neuron-rich cell culture model by differentiation of self-renewing human neuroprogenitor cells and a method to isolate a pure population of neurons by laser capture microdissection. We have used a combination of NGF, BDNF, DBC and retinoic acid for neuronal differentiation of NPCs. DBC is used to activate CREB, a transcription factor that enhances neurogenesis 12. Retinoic acid induces cell cycle exit and reduces the glial population 13. The method described in this study incorporates modifications over our previous report 14. We had earlier used the step of triturating the neurospheres to a single cell suspension and then seeding it in coated dishes. This can lead to inefficient neuronal differentiation in areas of low density (Figure 2E). We have now introduced the procedure of seeding four-day old small neurospheres (Figure 1B) to ensure contacts between differentiating neurons (Figure 1C). In addition, the differentiation supplement (Stem Cell Technologies) is replaced with B27 supplement (Invitrogen), 4-5 days after seeding. The current method yields 80-90% neurons consistently. These neurons with an extensive network of processes can be maintained in culture for 2-3 months, a distinct advantage over the neuronal model resulting from differentiation of human neuroblastoma cell lines. Immunostaining for several neuronal markers including NeuN, synapsin, acetylcholinesterase, synaptophysin and GAP43 were observed with differentiated NPCs (Figure 3). STAT3 and GFAP, markers of astrocytes were also detected in a small population of cells. By increasing the concentration of retinoic acid to 2-5 μM, the astrocyte population can be further reduced. However, such cultures cannot be maintained for a long duration because glial cells support neuronal survival by secretion of growth factors. We have observed that it is desirable to increase retinoic concentration 3-5 days before performing the experiments.
The heterogeneous nature of differentiated neurons presents challenges in terms of determining cell-type-specific gene expression profiling. Therefore, we have optimized the conditions for LCM of cultured neurons (Figure 4). In the current study, we differentiated NPCs on slides with PEN membrane inserts and performed LCM because this technique presented difficulties with neurons cultured on regular cell culture dishes as they are firmly attached. Although the objectives of LCM can be met by immunohistochemical analysis, LCM offers several advantages including the ability to amplify the signal at the RNA level, perform multiplex analysis, and accurate quantitation. When combined with microarray, LCM allows expression analysis of thousands of genes in selected cell types 15.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by Merit Review grant (NEUD-004-07F) from the Veterans Administration (to S.P).
Materials
| Name of Reagent/Material | Company | Catalog Number | Comments |
| Neuroprogenitor cells (NPCs) | Lonza | PT-2599 | |
| Neurocult NS-A human basal medium | Stem cell technology | 5750 | |
| Neurocult NS-A Proliferation supplement | Stem cell technology | 5753 | |
| Neurocult NS-A Differentiation supplement | Stem cell technology | 5754 | |
| B-27 Supplement (50x) | Invitrogen | 17504-044 | |
| Human epidermal growth factor | Stem cell technology | 2633 | |
| Fibroblast growth factor | Sigma | F0291 | |
| Brain Derived Neurotrophic Factor | Cell signaling | 3897S | |
| Nerve Growth Factor | Invitrogen | 13257-019 | |
| Dibutyryl cyclic AMP | Sigma | D-0627 | |
| poly-L-lysine | Sigma | P-5899 | |
| Mouse laminin | Sigma | L-2020 | |
| Retinoic acid | Sigma | R-2625 | |
| STAT3 antibody | Cell signaling | 9132 | |
| GFAP antibody | Cell signaling | 3670 | |
| GAP43 antibody | Transduction laboratories | 612262 | |
| BDNF antibody | Millipore | AB1534SP | |
| Acetyl cholinesterase antibody | Santz cruz | Sc-11409 | |
| Synaptophysin antibody | Abcam | ab18008-50 | |
| NeuN antibody | Chemicon | MAB377 | |
| Synapsin antibody | Novus | NB300-104 | |
| Anti mouse FITC | Jackson Immuno | 115-095-146 | |
| Research Laboratories | |||
| Anti Rabbit Cy3 | Jackson Immuno | 711-165-152 | |
| Research Laboratories | |||
| BSA | Sigma | A1653 | |
| Triton-X 100 | Acros | 21568-2500 | |
| Paraformaldehye | Fisher | 4042 | |
| Coverslip (Big circle cover slip) | Fisherbrand | 12-545-102 | |
| Mounting medium (Prolong Gold) | Invitrogen | P36930 | |
| Pen membrane | Applied biosystems | LCM0521 | |
| Histogene LCM frozen section staining kit | Applied biosystems | KIT0401 | |
| RiboAmp RNA Amplification kit | Applied biosystems | KIT0201 | |
| Picopure RNA Isolation kit | Applied biosystems | KIT0202 | |
| CapsureMacro LCM caps | Applied biosystems | LCM0211 |
Referenzen
- Doetsch, F. The glial identity of neural stem cells. Nat Neurosci. 6, 1127-1134 (2003).
- Galli, R., Gritti, A., Bonfanti, L., Vescovi, A. L. Neural stem cells: an overview. Circ. Res. 92, 598-608 (2003).
- Kim, S. U., de Vellis, J. Stem cell-based cell therapy in neurological diseases: a review. J. Neurosci. Res. 87, 2183-2200 (2009).
- Breier, J. M., et al. Neural progenitor cells as models for high-throughput screens of developmental neurotoxicity: state of the science. Neurotoxicol. Teratol. 32, 4-15 (2010).
- Christie, V. B., et al. Retinoid supplementation of differentiating human neural progenitors and embryonic stem cells leads to enhanced neurogenesis in vitro. J Neurosci Methods. 193, 239-245 (2010).
- Bonner, R. F., et al. Laser capture microdissection: molecular analysis of tissue. Science. 278, 1481-1483 (1997).
- Vincent, V. A., DeVoss, J. J., Ryan, H. S., Murphy, G. M. Analysis of neuronal gene expression with laser capture microdissection. J. Neurosci. Res. 69, 578-586 (2002).
- Robles, Y., et al. Hippocampal gene expression profiling in spatial discrimination learning. Neurobiol. Learn Mem. 80, 80-95 (2003).
- Su, J. M., et al. Comparison of ethanol versus formalin fixation on preservation of histology and RNA in laser capture microdissected brain tissues. Brain Pathol. 14, 175-182 (2004).
- Shimamura, M., Garcia, J. M., Prough, D. S., Hellmich, H. L. Laser capture microdissection and analysis of amplified antisense RNA from distinct cell populations of the young and aged rat brain: effect of traumatic brain injury on hippocampal gene expression. Brain Res. Mol. Brain Res. 122, 47-61 (2004).
- Pugazhenthi, S., Wang, M., Pham, S., Sze, C. I., Eckman, C. B. Downregulation of CREB expression in Alzheimer’s brain and in Abeta-treated rat hippocampal neurons. Mol. Neurodegener. 6, 60 (2011).
- Dworkin, S., Mantamadiotis, T. Targeting CREB signalling in neurogenesis. Expert Opin. Ther. Targets. 14, 869-879 (2010).
- Trujillo, C. A., et al. Novel perspectives of neural stem cell differentiation: from neurotransmitters to therapeutics. Cytometry A. 75, 38-53 (2009).
- Pugazhenthi, S., et al. Varicella-zoster virus infection of differentiated human neural stem cells. J. Virol. 85, 6678-6686 (2011).
- Kamme, F., et al. Single-cell microarray analysis in hippocampus CA1: demonstration and validation of cellular heterogeneity. J. Neurosci. 23, 3607-3615 (2003).

