Summary
Knowledge of the exact number of viable cells is required for many tissue culture manipulations. This protocol describes how to differentiate between live and dead cells and quantify cells using a hemacytometer. Although it describes counting human neural stem/precursor cells (hNSPCs), it can be used for other cell types.
Abstract
Knowledge of the exact number of viable cells in a given volume of a cell suspension is required for many routine tissue culture manipulations, such as plating cells for immunocytochemistry or for cell transfections. This protocol describes a straightforward and fast method for differentiating between live and dead cells and quantifying the cell concentration and total cell number using a hemacytometer. This procedure first requires detaching cells from a growth surface and resuspending them in media. Next, the cells are diluted in a solution of Trypan blue (ideally to a concentration that will give 20-50 cells per quadrant) and placed in the hemacytometer. Finally, averaging the counts of viable cells in several randomly selected quadrants, dividing the average by the volume of one 1 mm2 quadrant (0.1 μl) and multiplying by the dilution factor gives the number of cells per l. Multiplying this cell concentration by the total volume in μl gives the total cell number. This protocol describes counting human neural stem/precursor cells (hNSPCs), but can also be used for many other cell types.
Protocol
Passaging Human Neural Stem Cells
Note: Please refer to the Passaging Human Neural Stem Cells article on how to detach and resuspend the cells. https://www.jove.com/index/Details.stp?ID=263
Preparing the Cell Suspension for Counting
- Place 25 µl of 0.4% Trypan Blue solution and 20 µl of cell media into an eppendorf tube.
- Resuspend the cells to be counted by gently tapping the tube containing the cells in a known volume of media to create a homogenous suspension. Place 5 µl of cells into the eppendorf tube from the previous step. This step dilutes the cell concentration by a factor of 10.
- Place the cover glass on the hemacytometer, and load about 11-12 µl of the cell suspension.
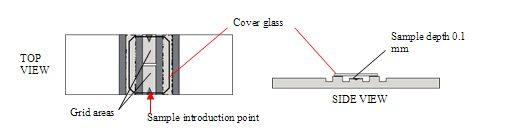
Counting Cells in the Hemacytometer
- Observe the entire grid of the hemacytometer in a phase contrast microscope. Focus on one quadrant of the grid (such as the one in red).
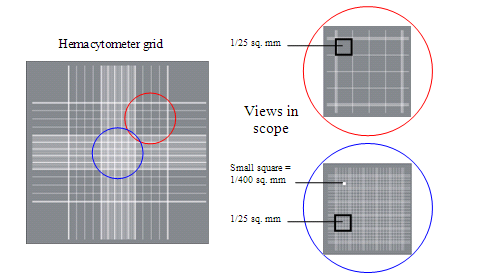
- Dead or dying cells will appear blue because their membrane has been damaged and are not able to exclude the Trypan blue dye. Viable cells will not appear blue and will be surrounded by a “halo” of light in phase contrast (will be “phase-bright”).
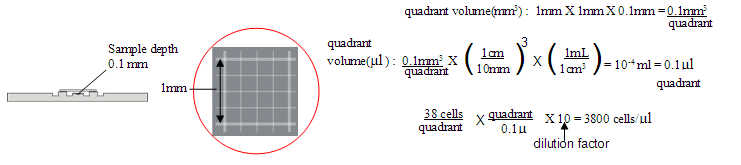
- Count the number of viable cells in one quadrant (area 1 mm2). You can also determine cell viability by dividing the number of viable cells by the total number of cells. Count cells in 3-4 random quadrants and determine the average number of cells per quadrant. Since the volume of a quadrant is known to be 10-4 ml or 0.1 µl, the concentration of cells can be determined
- You can use the following shortcut to determine the #cells/µl:
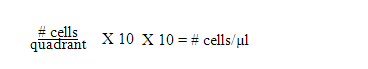
Total # of cells in tube =# cells/µl X µl in tube
Discussion
This protocol describes a straightforward and rapid way to determine the cell concentration for a variety of experiments involving cells. Using the shortcut described in 3d, one can quickly and accurately determine the cell concentration and total cell number in a given volume.
Acknowledgements
The authors would like to acknowledge Dr. Philip H. Schwartz of the National Human Neural Stem Cell Resource at the Children s Hospital, Orange County Research Institute for providing hNSPC cultures.
Materials
| Material Name | Typ | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Phase Contrast Hemacytometer | Tool | Hausser | 02-671-54 | Distributed by Fisher under the indicated catalog # |
| Trypan Blue Stain 0.4% | Reagent | Gibco | 15250-061 | Distributed by Invitrogen under the indicated catalog # |
Referenzen
- Marchenko, S., Flanagan, L. A. Passaging Human Neural Stem Cells. Journal of Visualized Experiments. 7, (2007).
- Caprette, D. a. v. i. d. R. Using a Hemacytometer. BioEd Online. , (2008).
- Caprette, D. a. v. i. d. R. Counting Chamber (Hemacytometer). BioEd Online. , (2008).

