Identifying Cell Surface Markers of Primary Neural Stem and Progenitor Cells by Metabolic Labeling of Sialoglycan
Summary
Presented here is a protocol that combines an in vitro neural-endothelial co-culture system and metabolic incorporation of sialoglycan with bioorthogonal functional groups to expand primary neural stem and progenitor cells and label their surface sialoglycoproteins for imaging or mass-spectrometry analysis of cell surface markers.
Abstract
Neural stem and progenitor cells (NSPCs) are the cellular basis for the complex structures and functions of the brain. They are located in specialized niches in vivo and can be isolated and expanded in vitro, serving as an important resource for cell transplantation to repair brain damage. However, NSPCs are heterogeneous and not clearly defined at the molecular level or purified due to a lack of specific cell surface markers. The protocol presented, which has been previously reported, combines a neural-endothelial co-culture system with a metabolic glycan labeling method to identify the surface sialoglycoproteome of primary NSPCs. The NSPC-endothelial co-culture system allows self-renewal and expansion of primary NSPCs in vitro, generating a sufficient number of NSPCs. Sialoglycans in cultured NSPCs are labeled using an unnatural sialic acid metabolic reporter with bioorthogonal functional groups. By comparing the sialoglycoproteome from self-renewing NSPCs expanded in an endothelial co-culture with differentiating neural culture, we identify a list of membrane proteins that are enriched in NSPCs. In detail, the protocol involves: 1) set-up of an NSPC-endothelial co-culture and NSPC differentiating culture; 2) labeling with azidosugar per-O-acetylated N-azidoacetylmannosamine (Ac4ManNAz); and 3) biotin conjugation to modified sialoglycan for imaging after fixation of neural culture or protein extraction from neural culture for mass spectrometry analysis. Then, the NSPC-enriched surface marker candidates are selected by comparative analysis of mass spectrometry data from both the expanded NSPC and differentiated neural cultures. This protocol is highly sensitive for identifying membrane proteins of low abundance in the starting materials, and it can be applied to marker discovery in other systems with appropriate modifications
Introduction
Neural stem cells are defined as a multipotent cell population that can self-renew to maintain a stem cell pool and differentiate into neurons and glia. They are the major cell types in the nervous system and may offer great therapeutic potential in regenerative medicine through cell transplantation into diseased and injured brains1,2. As development proceeds, the neural stem cell population becomes heterogenous3,4, and the proportion of neural stem cells in the brain gradually decreases5. Generally speaking, embryonic neural stem cells and other neural progenitor cells, collectively called neural stem and progenitor cells (NSPCs), are located in the germinal zones, the ventricular zone, and the subventricular zone in mice6. In the embryonic brain, neural stem cells generate neurons directly or indirectly through intermediate progenitor cells (IPCs), and in some species through the outer subventricular zone progenitors (oRGs)7,8. The specific molecular signature, morphology, location in the stem cell niche, and differentiation potential all determine the role of each subtype in brain organogenesis and clinical applications9. However, the currently available cell surface markers cannot unequivocally discriminate and purify different subtypes of NSPCs, limiting the understanding and utilization of these subtypes.
The identification of primary NSPCs surface markers is limited by three major hurdles. The first one is the limited cell number of NSPCs in the tissue, making it difficult to prepare cell surface protein samples for common mass spectrometry analysis. The second limitation is the difficulty in producing pure cell subtypes for generating subtype-specific membrane protein data. Finally, the third challenge is the low ratio of cell surface proteins in whole cell proteins, which hampers their detection sensitivities by mass spectrometry analysis.
To overcome these problems, we developed a chemoproteomic approach to selectively enrich and identify cell surface proteins in primary NSPCs by metabolically labeling the sialoglycoproteins10. To generate a sufficient number of NSPCs, we took advantage of an established protocol to expand and maintain primary embryonic NSPCs in undifferentiated states in vitro, by co-culturing NSPCs with mouse brain endothelial cell lines using a permeable support matrix insert (e.g., transwell) system11. In contrast, NPSCs cultured alone without endothelial cells generate differentiated progeny11,12. Thus, protein samples from these two culture systems can be comparatively analyzed to identify proteins that are differentially expressed in NSPCs and differentiated neurons. As most cell surface proteins are modified by sialic acid13, unnatural sialic acid precursor analog N-azidoacetylmannosamine-tetraacylated (Ac4ManNAz) was used to hijack the intrinsic metabolic pathway so that endogenous, newly synthesized sialoglycans are labeled with azido groups, generating a chemical handle14. Through azido-alkyne-mediated bioorthogonal reactions, which conjugate biotin to sialoglycans, cell surface proteins can be visualized and enriched for proteomic identification through a streptavidin-coupled fluorophore or matrix14.
Here, we perform staining of SDS-PAGE gel analysis of the surface sialoglycoproteome from NSPCs expanded in an endothelial co-culture and differentiating cells in a non-co-culture system. We also selectively purify surface sialoglycoproteome in the two culture systems for proteomic comparison. Our protocol, compared with the traditional centrifugation-based cell surface purification protocols15, increases extraction efficacy by reducing the surface protein extraction procedures through specific tag conjugation and affinity purification. Meanwhile, it increases the extraction purity of cell surface proteins based on the premise that sialylation happens mostly at the cell surface proteins. Although endothelial factors cannot completely block differentiation of expanded NSPCs, the comparative study between a co-culture and differentiated culture provides a convenient method to pinpoint stem cell-enriched surface proteins without the need to analyze proteins from NPCs purified by FACS16. We believe this approach can be applied to studies of surface proteins in other systems with the appropriate modifications.
Protocol
All animal protocols used in this study were approved by the IACUC (Institutional Animal Care and Use Committee) of Tsinghua University and performed in accordance with guidelines of the IACUC. The laboratory animal facility at Tsinghua University has been accredited by the AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care International). For staging of embryos, mid-day of the vaginal plug identified was calculated as embryonic day 0.5 (E0.5).
NOTE: All cells are cultured in the cell incubator under conditions of 37 °C and 5% CO2.
1. Preparation of Mouse Endothelial Culture in Permeable Support Inserts
NOTE: BEND3 cells are maintained according to manufacturer's instructions.
- Prepare BEND3 cell medium (BM) by adding 50 mL of FBS and 5 mL of penicillin-streptomycin into 500 mL of DMEM and mix well.
- Aspirate the medium from the dish and wash the BEND3 cells culture with 1 mL of PBS once. Add 1 mL of 0.25% Trypsin-EDTA into the cells and incubate the cells for 4 min at 37 °C.
- Add 1 mL of BM into the cells to neutralize Trypsin-EDTA and pipette up and down gently to completely dissociate the cells. Transfer the cell suspension into a new 15 mL conical tube and pellet by centrifugation at room temperature (RT) for 5 min at 400 x g.
- Aspirate the supernatant from the tube and resuspend the cells with 9 mL of fresh BM, then add 1 mL of cell suspension into one permeable support insert. Add another 2 mL of fresh BM per well at the bottom chamber of the matrix. Continue to culture the cells for one day.
2. Preparation of Mouse Primary Cortical NSPCs Culture
- Preparation of culture plate, papain digestion medium, and cortical adherent culture medium (AM)
- Coat 6-well plates with poly-L-lysine (PLL) by adding 1 mL of PLL solution per well into 6-well plates. Then, incubate the plates at RT for 30 min.
- Transfer the PLL solution into a 15 mL conical tube. Wash the plates 3 times with double distilled water. Airdry the plates and put them aside until use.
- Prepare the papain digestion medium by adding 50 U of papain, 50 µL of L-glutamine, and 50 µL of 100 mg/mL acetyl-L-cysteine into 5 mL of DMEM. Mix the medium briefly and warm it to 37 °C for 30 min for enzyme activation.
- Prepare the cortical cell adherent culture medium (AM): add 500 µL of L-glutamine, 500 µL of sodium pyruvate, 500 µL of 100 mg/mL N-acetyl-L-Cysteine, 500 µL of N2, 1 mL of B27, and 5 µL of 100 µg/mL bFGF into 50 mL of DMEM. Mix the medium well and warm it to 37 °C before use.
- Preparation of primary cerebral cortical cells and subsequent plating
- Sacrifice an E10.5 timed pregnant mouse by cervical dislocation.
NOTE: At E10.5, a majority of cells are proliferating NSPCs in the cerebral cortex, giving rise to large clones of progeny in vitro. - Sterilize the abdomen by 75% ethanol. Use fine scissors and micro-serrated forceps to open the abdomen by cutting the skin and underlying muscle along the right side of the middle line. Remove the uterus from the abdominal cavity gently with serrated forceps and cut it out from the abdominal cavity with fine scissors.
- Wash the uterus with 40 mL of pre-chilled HBSS in 10 cm Petri dish. Then, transfer the uterus into a new 10 cm Petri dish and wash it again with 40 mL of pre-chilled HBSS.
- Transfer the uterus into a new 10 cm Petri dish with 40 mL of pre-chilled HBSS. Remove the embryos from the uterus and amniotic membrane, then cut the heads of the embryos off from the trunks with Jewelers microforceps.
- Wash the heads with 40 mL of pre-chilled HBSS and transfer the heads to a new 10 cm Petri dish with 40 mL of pre-chilled HBSS. Use Jewelers microforceps to peel away skin and cartilage covering the brains, then cut the cerebral cortices off and collect them in a 15 mL conical tube with pre-chilled HBSS.
- Pellet the cortices by centrifugation for 3 min at 4 °C and 300 x g. Aspirate the supernatant from the tube, then add activated papain digestion medium and 15 µL of 4 mg/mL DNase I into the tissue pellet.
- Resuspend the tissue pellet briefly by gentle vortexing. Incubate the tissue at 37 °C for 30 min. During this time, loosen the tissue by brief vortexing every 10 min.
NOTE: At the end of the digestion, there should be no visible tissue pieces in the tube. - Pellet the cortical cells by centrifugation for 10 min at 4 °C and 450 x g. Aspirate the supernatant from the tube and wash the cell pellet with pre-chilled DMEM. Repeat this step once.
NOTE: During the digestion and washing, take caution not to pipette the tissues and cell pellet roughly to avoid damaging the cells with a strong shearing force. - Aspirate the supernatant from the tube then add 1.5 mL of pre-chilled HBSS into the tube. Dissociate the cortical cell pellet into single cells with gentle pipetting. Count the cell number with a hemocytometer.
- Add 2 mL of AM and 2 x 104 cortical cells per well into 6-well plates. Incubate the plate at 37 °C and 5% CO2 for 3 h to let the cells attach to the plate.
- Sacrifice an E10.5 timed pregnant mouse by cervical dislocation.
3. Set-up of Neural-endothelial Co-culture and Ac4ManNAz Labeling System
- One day after plating BEND3 cells in the inserts, gently aspirate the medium in the bottom chamber first, then the inserts. Wash the enface of the inserts 3 times with pre-warmed DMEM. Wash the outer surface of the inserts by rinsing with pre-warmed DMEM.
- Add 1 mL of pre-warmed AM into one insert, then transfer the inserts into the wells with primary cortical cells. Incubate the co-culture at 37 °C and 5% CO2 for 12 h.
- Dissolve Ac4ManNAz in DMSO to achieve a stock concentration of 200 mM. 12 h after setting up the neural-endothelial co-culture, add 1 µL of Ac4ManNAz stock per bottom chamber and 0.5 µL of stock per insert into the co-culture. Shake the plates immediately and gently to mix the medium well. For the control cells, add equal volume of DMSO.
- Culture the cells for another 5 days at 37 °C and 5% CO2. Prepare the AM with 10x bFGF as refeeding medium (RM). During this time, add 100 µL of RM per insert and 200 µL of RM per bottom chamber to refeed the endothelial and neural cells every other day. During the refeeding, do not supply Ac4ManNAz or DMSO into the culture.
4. Immunofluorescent Staining of Sialoglycoproteins in Expanded Primary NSPCs and Differentiated Neurons
- Prepare BTTAA-CuSO4 complex 1 30x stock containing 1.5 mM CuSO4 and 9 mM BTTAA in double-distilled water. Prepare freshly biotin-conjugated buffer 1 containing 50 µM biotin-alkyne, 2.5 mM sodium ascorbate, and 1x BTTAA-CuSO4 complex in PBS.
- Remove the inserts from the co-culture plates. Aspirate the culture medium from the bottom wells and wash the neural cells once with pre-warmed PBS.
- Aspirate the PBS from the wells. Add 1 mL of pre-chilled 4% paraformaldehyde PBS solution per well into the cells and fix the cells at RT for 10 min. Then, wash the cells 3 times with pre-chilled PBS.
- Aspirate PBS from the wells and add 1 mL of freshly prepared biotin-conjugated buffer 1 per well into the cells. Incubate the cells at RT for 10 min.
- Aspirate the reaction buffer from the wells. Wash the cells 3 times with PBS. Prepare the staining buffer containing 1% FBS and 1 µg/mL Alexa Fluor 647-streptavidin. Add 1 mL of staining buffer per well into the cells and incubate the cells at RT for 30 min.
- Aspirate the staining buffer from the wells and washed cells 3 times with pre-chilled PBS. Prepare the blocking buffer containing 5% BSA and 0.3% non-ionic detergent-100 in PBS. Add 1 mL of blocking buffer per well into the cells and incubate at RT for 10 min.
- Prepare a primary antibody solution by diluting the anti-nestin and anti-β-tubulin III antibodies together into the blocking buffer at ratios of 1:20 and 1:1,000, respectively. Remove the blocking buffer from the wells and add 1 mL of primary antibody solution per well into the cells. Incubate the cells at 4 °C overnight.
- Remove the primary antibody solution from the wells. Wash the cells 3 times with pre-chilled PBS. Prepare a secondary antibody solution by diluting Alexa Fluor 488 goat anti-mouse IgG1, Alexa Fluor 546 goat anti-mouse IgG2b, and DAPI together into blocking buffer at a dilution of 1:1,000. Aspirate the PBS from the wells and add 1 mL secondary antibody solution per well into cells. Incubate the cells at RT for 2 h.
- Aspirate the antibody solution from the wells and wash the cells 3 times with pre-chilled PBS. Afterwards, the cells are ready for image capture.
5. Purification of Sialoglycoproteins from Expanded Primary NSPCs and Differentiated Neurons
- Prepare BTTAA-CuSO4 complex 2 15x stock containing 1.5 mM CuSO4 and 3 mM BTTAA in double distilled water. Prepare protein resuspension buffer A containing 4% SDS and 10 mM EDTA in double distilled water; protein resuspension buffer B containing 150 mM NaCl, 50 mM triethanolamine, and 1% polyoxyethylene oleyl ether (e.g., Brij97) in double distilled water with pH 7.4. Before use, mix buffer A:buffer B = 1:8 (vol/vol) to prepare the full protein resuspension buffer. Prepare protein washing buffer 1 containing 2% SDS in PBS; protein washing buffer 2 containing 8 M urea in 250 mM ammonium bicarbonate (ABC); and protein washing buffer 3 containing 2.5 M NaCl in PBS.
- Remove the inserts from the co-culture plates. Aspirate the culture medium from the bottom wells and wash the neural cells once with pre-chilled PBS.
- Aspirate the PBS from the wells and add 200 µL of pre-chilled RIPA buffer per well into the plates. Incubate the plates on ice for 5 min. Collect the protein lysis into 1.5 mL tubes. Pellet the cell debris by centrifugation for 10 min at 4 °C and 12,000 x g.
- Transfer the supernatant into new 1.5 mL tubes. Determine protein concentration with the BCA kit according to the manufacturer's instructions. Adjust the protein concentration to 1 mg/mL.
- Add 100 µM alkyne-biotin, 2.5 mM sodium ascorbate, and 1x BTTAA-CuSO4 complex 2 to 1 mL of protein lysis and mix the solution well. Incubate the mix at RT for 1 h.
- Transfer the reaction solution into 20 mL of pre-chilled methanol in a 50 mL conical tube. Mix well and incubate at -30 °C overnight to precipitate the proteins.
- Pellet the protein precipitates by centrifugation for 15 min at 4 °C and 4,500 x g. Wash the protein pellet twice with 20 mL of pre-chilled methanol. Aspirate the supernatant from the tube. Resuspend the protein pellet with 4 mL of protein resuspension buffer and transfer the protein resuspension into a new 15 mL conical tube.
- Take 50 µL of streptavidin beads and wash them 3 times with PBS. Add the washed beads into the protein resuspension. Incubate the solution at 4 °C for 3 h on a vertical rotator at a rotation speed of 20 rpm.
- Wash the beads sequentially with 6 types of buffers: protein washing buffer 1, protein washing buffer 2, protein washing buffer 3, 0.5 M ABC, 0.25 M ABC and 0.05 M ABC.
- After washing, resuspend the beads with 20 µL of PBS and transfer the beads into a new 1.5 mL tube. Add 20 µL of 2x protein loading buffer into the beads and treat at 95 °C for 10 min. The protein samples should then be subjected to SDS-PAGE and stained with Coomassie brilliant blue R-250 according to the manufacturer's instructions. Cut the proteins in gel as indicated by Coomassie brilliant blue R-250 for mass spectrometry analysis.
Representative Results
The whole procedure for in vitro expansion and metabolic labeling of primary embryonic NSPCs takes 6 days (Figure 1A). Quality of the BEND3 cell line and freshly isolated primary NSPCs are key to a successful experiment. BEND3 cells are the source of soluble factors that stimulate self-renewal and proliferation of NSPCs. It should be ensured that the BEND3 cells are free of any contamination and divide actively with minimal cell death before co-culturing with neural cells. The primary NSPCs must be carefully prepared to avoid excess damage during dissociation. Damaged NSPCs may still grow and differentiate; however, they are not able to respond to endothelial stimuli well to maintain stemness and expand. Extra caution should be taken to be aseptic during cell culturing, as the protocol does not suggest addition of antibiotics to the primary culture medium.
Successful endothelial co-culture will lead NSPCs to form large, sheet-like clones. Such featured clone shapes become evident at day 4 and are very typical at day 6. Within the clones, the cells maintain close contact with each other. Immunostaining with antibodies against the NSPC marker Nestin and the neuronal marker β-tubulin III should reveal that in the clone, most of the cells are Nestin+ NSPCs and very few are β-tubulin III+ neuronal cells. In contrast, the percentage of Nestin+ cells and β-tubulin III+ neuronal cells in clone formed in non-co-culture system are nearly the same (Figure 1B, 1D, and 1E).
The chemical reporter, Ac4ManNAz, is a metabolic analog and can be incorporated into the intrinsic protein sialylation pathway. High doses of Ac4ManNAz are toxic to cells. For each specific type of cell, the labeling concentration of Ac4ManNAz should be pre-tested to achieve the highest labeling efficiency without significant cytotoxicity. Here, the optimized labeling concentration of Ac4ManNAz for primary NSPC is 100 µM. Combinatory evaluation of cell death indicated by cellular and nuclei morphology suggests this labeling concentration does not cause obvious cytotoxic effects and is able to efficiently label NSPCs (Figure 1C and 1D). The clonal morphology, self-renewal, and differentiation potential of NSPCs in both the endothelial co-culture and non-co-culture system are not affected (Figure 1C, 1D, and 1E).
The successful labeling of NSPCs by Ac4ManNAz can be examined after conjugating biotin to a culture mediated by a bioorthogonal reaction between azide and alkyne. Every cell in the Ac4ManNAz-labeled culture is stained and visualized with Alexa Fluor 647-streptavidin. No cell is positive for Alexa Fluor 647-streptavidin staining in the DMSO control group. In addition, protein samples prepared from the Ac4ManNAz-labeled culture by biotin conjugation and streptavidin beads purification show strong Coomassie brilliant blue staining signal in SDS-PAGE gels. Meanwhile, there were only staining background and nonspecific binding signals in the lanes loaded with protein samples from the DMSO control group. This also indicates the efficient labeling of NSPCs by Ac4ManNAz (Figure 1F).
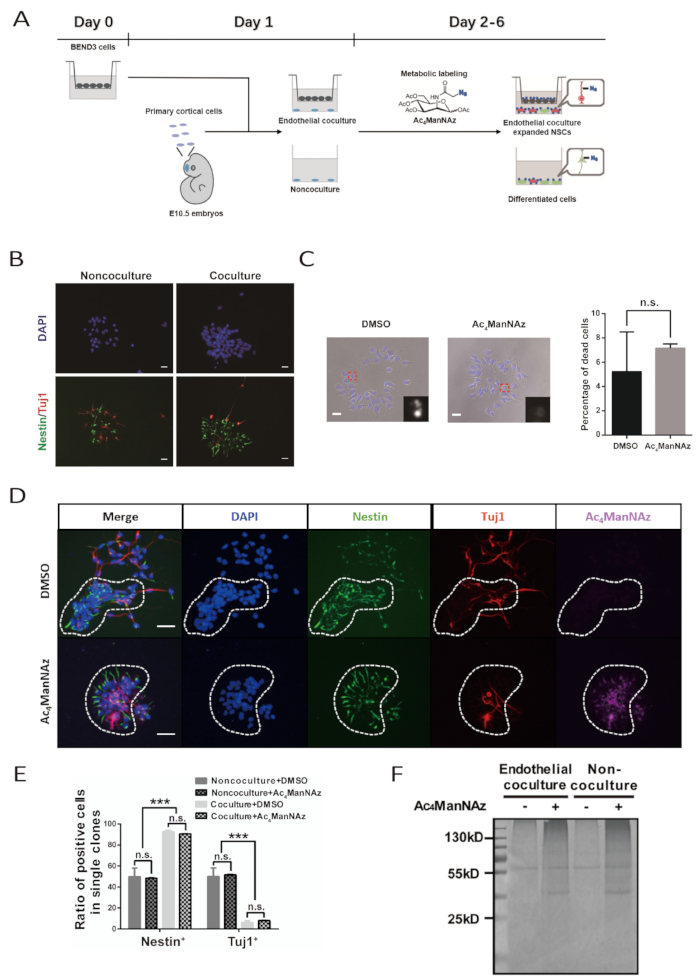
Figure 1: Identification of cell surface markers for primary NSPCs assisted by endothelial co-culture system and metabolic sialoglycan labeling. (A) Schematic of the workflow for the protocol. This figure has been modified from Bai et al.10. The BEND3 cells are seeded into matrix inserts on D0. The preparation of primary cortical NSPCs and set up of co-culture system are performed on D1. Metabolic labeling of culture lasts from D2 to D6. Culture refeeding is carried on D3 and D5. (B) The immunofluorescent images for clones formed by primary NSPCs after 5-day culture with or without endothelial cells. Scale bar indicates 20 µm. (C) Bright-field images for clones formed by primary NSPCs after a 5-day culture with Ac4ManNAz or DMSO. The nuclei were counterstained by DAPI. The scale bar indicates 20 µm. The error bar indicates SEM (n.s. = not significant). (D) The immunofluorescent images for NSPC formed clones in the endothelial co-culture with Ac4ManNAz or DMSO. Dashed circle demarcates a single neural clone. The scale bar indicates 50 µm. (E) Quantification of NSPCs and differentiated neurons in clones formed by NSPCs in endothelial co-culture and non-co-culture system with Ac4ManNAz labeling or DMSO control. The error bar indicates SEM (***p < 0.0005; n.s. = not significant). (F) Coomassie brilliant blue staining of proteins purified by streptavidin beads from neural cells labeled with Ac4ManNAz or DMSO in endothelial co-culture and nonco-culture system. The 55 kD band in control labelling groups represents nonspecific binding proteins. (B, C, E and F) corresponding to this protocol have been adapted from Bai et al.10. Please click here to view a larger version of this figure.
Discussion
Surface markers are commonly used to label and purify specific cell types in vitro and in vivo17,18. Discovery of surface markers contributes greatly to regenerative medicine and stem cell researches by providing molecular tools to selectively enrich a stem cell population from normal or pathological tissues and culture dishes, offering a purified cell resource for clinical use or study of biological properties. However, progress in developing surface markers for neural stem cell research has been slow due to the difficulty in isolating stem cells from primary tissues. The protocol described here is based on a simplified in vitro platform. By comparing primary NSPCs expanded by an endothelial co-culture to a differentiating neural culture, proteins differentially expressed in expanded NSPCs are highlighted and allow for further identification. Our protocol also provides an alternative strategy to purify cell surface proteins by hijacking the intrinsic metabolic pathway to label sialoglycan with bioorthogonal groups. Compared with traditional protocols for purifying cell surface proteins, the advantages of this protocol are underpinned by two specific features: 1) the prevalence of sialylation on cell surface proteins ensures maximal coverage of the cell surface proteome, and 2) the reaction specificity between the bioorthogonal group and its ligands grants purity of the acquired surface proteome. Thus, our protocol results in a more sensitive proteomic analysis in the case of less starting materials. We have demonstrated the feasibility of this protocol in primary NSPCs surface markers. With the appropriate modifications on expanding stem cells in vitro, this chemoproteomic approach can be compatible with identifying surface markers of other stem cell types. It is noteworthy that as Ac4ManNAz is per-O-acetylated it could lead to artificial S-glycosylation. The use of unacetylated unnatural sugars can avoid the artifact formation and improve the specificity and validity of metabolic glycan labeling in living cells19.
Preparation of primary cortical neural progenitor cells and endothelial cells are critical steps of the protocol. First, when digesting embryonic cortical tissues, the digestion time, amount of enzyme, and strength of handling must be carefully controlled. Excessive digestion and mechanical shearing forces will damage the integrity of plasma membrane and cell surface receptors that mediate signal transduction for cell survival and growth, and they will also disturb the responsiveness of NSPCs to the stimulation of endothelial cells and their self-renewal ability. To achieve proper digestion, experimenters must activate the papain fully and stop the digestion as soon as the tissue blocks disappear. Second, BEND3 cells must be maintained in a healthy state to support the secretion. It is recommended to use BEND3 cell batches with fewer passages and passage the cells before they reach 100% confluence. This will prevent cell cycle arrest and senescence caused by DNA damage accumulated during passaging or by overcrowded contact between cells.
High throughput sequencing technology boosts the identification of cell surface markers through analyzing RNA expression, especially for cell types including tissue stem cells, which are often present in vivo in amounts too small to perform proteome analysis by mass spectrometry. Even though RNA-seq analysis can identify genes specifically expressed in NSPCs, it may not truly reflect protein expression levels, because RNA expression is not always consistent with protein expression20. In addition, non-protein biomolecules that can work as surface markers are not able to be detected by transcriptomic studies. For example, oligosaccharide Lewis X is a well-known surface maker widely used to label human embryonic stem cells and NSPCs, even though it can be associated with multiple proteins21. Therefore, direct mass spectrometry analysis is not yet substitutable, and the development of methods that can make mass spectrometry analysis more feasible and convenient is of great interest for future studies.
In addition to sialylation, other types of post-translational protein modifications play an important role in regulating functions of modified proteins. These modifications affect protein properties such as the conformation, half-life, and subcellular localization22,23. Several protein modifications have cell type specificity24,25,26. With the growing contents of the chemical toolbox, more modification types are amenable to metabolic labelling with chemical reporters27. Hence, the chemical approach described here can be used for studying other differences in protein modification between stem cells and differentiated cells, illustrating the molecular mechanisms behind maintenance of stem cell properties and differentiation regulation.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
Figure 1B, 1C, 1E and 1F are reproduced from Bai et al.10 with permission from the Royal Society of Chemistry. We thank Yi Hao in X. C.'s lab for figure editing. This work is supported by the National Natural Science Foundation of China (No. 91753206 to Q. S. and X. C., No. 31371093 to Q. S., and Nos. 21425204 and 21672013 to X. C.).
Materials
| BEND3 | ATCC | CRL-229 | |
| DMEM | Gibco | 11960044 | |
| L-glutamine | Gibco | 25030081 | 1% |
| Sodium pyruvate | Sigma | P5280 | 1% |
| N2 supplement | Gibco | 17502048 | 1 to 100 |
| N-acetyl-L-cysteine | Sigma | A7250 | 1 mM |
| Papain | Worthington | LS003726 | 10 U/mL |
| B27 supplement | Gibco | 17504044 | 1 to 50 |
| Poly-L-lysine | Sigma | P4707 | |
| basic Fibroblast growth factor | Gibco | PHG0261 | 10 ng/mL |
| Penicillin-Streptomycin | Gibco | 15140122 | 1% |
| Fetal bovine serum | Gibco | 10099141 | 10% |
| HBSS | Gibco | 14175095 | |
| Tripsin-EDTA, 0.25% | Gibco | 25200056 | |
| DPBS | Gibco | 14190094 | |
| Transwell | Corning | 3450 | |
| Paraformaldehyde | Sigma | 158127 | 4% |
| Sucrose | Sangon | A100335 | |
| DAPI | Gibco | 62248 | |
| RIPA buffer | Thermo Scientific | 89900 | |
| SDS-PAGE loading buffer 2X | Solarbio | P1018 | |
| 6-well plate | Corning | 3335 | |
| Tris-Glycine protein gel | invitrogen | xp00100box | |
| mouse monoclonal anti-Nestin | Developmental Study Hybridoma Bank | Rat-401 | 1 to 20 |
| mouse monoclonal anti-beta-tubulin III | Sigma | T8860 | 1 to 1000 |
| Alexa Fluor 488 goat anti-mouse IgG1 | invitrogen | A-21121 | 1 to 1000 |
| Alexa Fluor 546 goat anti-mouse IgG2b | invitrogen | A-21143 | 1 to 1000 |
| Albumin Bovine V | Amresco | 0332 | |
| Triton X-100 | Amresco | 0694 | |
| BCA assay kit | Thermo Scientific | 23225 | |
| dimethyl sulfoxide | Sigma | D2650 | |
| Brij97 | Aladdin | B129088 | |
| CuSO4 | Sigma | 209198 | |
| alkyne-biotin | Click Chemistry Tools | TA105 | |
| BTTAA | Click Chemistry Tools | 1236 | |
| Ac4ManNAz | Click Chemistry Tools | 1084 | 100 µM |
| 9AzSia | synthesized in lab | ||
| sodium ascorbate | Sigma | A4034 | |
| Methanol | Sigma | 34860 | |
| EDTA | Sangon | A100322 | |
| NaCl | Sangon | A100241 | |
| SDS | Sangon | A100227 | |
| Alexa Flour 647-conjugated streptavidin | invitrogen | S21374 | 1 to 1000 |
| Triethanolamine | Sigma | V900257 | |
| Dynabeads M-280 Streptavidin | invitrogen | 60210 | |
| ammonium bicarbonate | Sigma | 9830 | |
| Coomassie Brilliant Blue R-250 | Thermo Scientific | 20278 | |
| Isoflurane | RWD Life Science Co. | 970-00026-00 | |
| DNase I | Sigma | DN25 | 12 µg/mL |
| urea | Sigma | U5378 |
Referencias
- Weissman, I. L. Stem Cells: Units of Development, Units of Regeneration, and Units in Evolution. Cell. 100, 157-168 (2000).
- Gage, F. H., Temple, S. Neural Stem Cells: Generating and Regenerating the Brain. Neuron. 80, 588-601 (2013).
- Gal, J. S. Molecular and Morphological Heterogeneity of Neural Precursors in the Mouse Neocortical Proliferative Zones. Journal of Neuroscience. 26, 1045-1056 (2006).
- Kawaguchi, A., et al. Single-cell gene profiling defines differential progenitor subclasses in mammalian neurogenesis. Development. 135, 3113-3124 (2008).
- Temple, S. The development of neural stem cells. Nature. 414, 112-117 (2001).
- Kwan, K. Y., Sestan, N., Anton, E. S. Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development. 139, 1535-1546 (2012).
- Kriegstein, A., Alvarez-Buylla, A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annual Review of Neuroscience. 32, 149-184 (2009).
- Wang, X., Tsai, J. W., LaMonica, B., Kriegstein, A. R. A new subtype of progenitor cell in the mouse embryonic neocortex. Nature Neuroscience. 14, 555-561 (2011).
- Taverna, E., Götz, M., Huttner, W. B. The Cell Biology of Neurogenesis: Toward an Understanding of the Development and Evolution of the Neocortex. Annual Review of Cell and Developmental Biology. 30, 465-502 (2014).
- Bai, Q. R., Dong, L., Hao, Y., Chen, X., Shen, Q. Metabolic glycan labeling-assisted discovery of cell-surface markers for primary neural stem and progenitor cells. Chemical Communications. 54, 5486-5489 (2018).
- Shen, Q., et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 304, 1338-1340 (2004).
- Qian, X., et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 28, 69-80 (2000).
- Varki, A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 446, 1023-1029 (2007).
- Cheng, B., Xie, R., Dong, L., Chen, X. Metabolic Remodeling of Cell-Surface Sialic Acids: Principles, Applications, and Recent Advances. ChemBioChem. 17, 11-27 (2016).
- Lin, S. H., Guidotti, G. Purification of Membrane Proteins. Methods in Enzymology. 463, 619-629 (2009).
- Schmidt, J. R., et al. Pilot Study on Mass Spectrometry-Based Analysis of the Proteome of CD34+CD123+ Progenitor Cells for the Identification of Potential Targets for Immunotherapy in Acute Myeloid Leukemia. Proteomes. 6, (2018).
- Crisan, M., Dzierzak, E. The many faces of hematopoietic stem cell heterogeneity Development. Development. 144, 4195-4195 (2017).
- Uchida, N., et al. Direct isolation of human central nervous system stem cells. Proceedings of the National Academy of Sciences of the United States of America. 97, 14720-14725 (2000).
- Qin, W., et al. Artificial Cysteine S-Glycosylation Induced by Per-O-Acetylated Unnatural Monosaccharides during Metabolic Glycan Labeling. Angewandte Chemie International Edition. , (2018).
- Gry, M., et al. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics. 10, 365 (2009).
- Hennen, E., et al. A LewisX Glycoprotein Screen Identifies the Low Density Lipoprotein Receptor-related Protein 1 (LRP1) as a Modulator of Oligodendrogenesis in Mice. Journal of Biological Chemistry. 288, 16538-16545 (2013).
- Seet, B. T., Dikic, I., Zhou, M. M., Pawson, T. Reading protein modifications with interaction domains. Nature Reviews Molecular Cell Biology. 7, 473-483 (2006).
- O’Brian, C. A., Chu, F. ReviewPost-translational disulfide modifications in cell signaling—role of inter-protein, intra-protein, S-glutathionyl, and S-cysteaminyl disulfide modifications in signal transmission. Free Radical Research. 39, 471-480 (2005).
- Williamson, A. J. K., Whetton, A. D. The requirement for proteomics to unravel stem cell regulatory mechanisms. Journal of Cellular Physiology. 226, 2478-2483 (2011).
- Christensen, B., et al. Cell Type-specific Post-translational Modifications of Mouse Osteopontin Are Associated with Different Adhesive Properties. Journal of Biological Chemistry. 282, 19463-19472 (2007).
- Yanagisawa, M., Yu, R. K. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 17, 57R-74R (2007).
- Best, M. D. Click Chemistry and Bioorthogonal Reactions: Unprecedented Selectivity in the Labeling of Biological Molecules. Bioquímica. 48, 6571-6584 (2009).

