同时进行机电刺激增强细胞心肌成功能的潜能
Summary
在这里, 我们提出了一个协议, 训练细胞群使用电子和机械刺激模拟心脏生理。这种机电刺激增强了治疗细胞的心肌潜能, 是进一步进行细胞治疗、疾病建模和药物筛选的有希望的策略。
Abstract
心血管疾病是发达国家的主要死因。因此, 对有效的心脏细胞疗法的需求促使干细胞和生物工程领域的研究人员在体外开发高保真的人心肌, 用于基础研究和临床应用。然而, 心脏细胞的未成熟表型是获得功能模仿成人心肌的组织的一个限制, 而成年心肌主要以机械和电信号为特征。因此, 该协议的目的是通过机电刺激、重述生理参数来制备和成熟靶细胞群。心脏组织工程正在向更多的生物方法发展, 因此, 基于生物物理刺激的策略正在获得动力。为此目的开发的设备是独特的, 允许单独或同时进行电气和机械刺激, 经过仔细的表征和验证。此外, 尽管该方法已针对这种刺激器和特定的细胞群进行了优化, 但它可以很容易地适应其他设备和细胞系。这里的结果提供了在机电刺激后细胞群心脏承诺增加的证据。运动刺激细胞表现出增加的主要心脏标记表达, 包括早期, 结构, 和钙调节基因。这种细胞调理可用于进一步再生细胞治疗、疾病建模和高通量药物筛选。
Introduction
心功能是基于电激励和机械收缩的耦合。简单地说, 心肌细胞间的连接允许电信号的传播产生几乎同步收缩的心脏, 泵血系统和通过肺系统。因此, 心脏细胞经历了调节基因表达和细胞功能的电和机械力。因此, 许多团体试图开发模仿心脏生理环境的培养平台, 以了解机械和电刺激对心脏发育、功能和成熟的作用。体外电和机械刺激在心脏组织工程中得到了广泛的应用, 以增强功能特性, 提高细胞成熟, 或改善细胞细胞耦合和钙处理1,2,3 个,4 个,5,6,7.,8,9,10,11,12,13,14,15,16,17,18,19,20,21. 然而, 由于开发刺激器和协议的挑战, 以及由于强制性优化 22, 同步机电调节仍未得到开发22。
初步工作将机电刺激作为电刺激和介质灌注的结合;然而, 流动不涉及应变为基础的变形典型的心室充盈 23,24,25。后来, 更多的生理方法结合电刺激与物理变形或拉伸, 以模仿等体积收缩 26,27,28,29,30 ,31。feng 等人介绍了2005年首次演示机电刺激, 报告心肌细胞大小和收缩特性增强26。wang 等人接受了 5-azacytidine 预处理的间充质干细胞, 并同时进行了电气和机械调理, 改善了再细胞化、细胞活力、心脏分化和组织重塑27。自这些出版物以来, 更多的团体报告了细胞单层或工程组织的机电刺激情况 (例如, black28、vunjak-novakovic29、31和我们的第30组)。第一个被适应的细胞在体内测试 30。简单地说, 摩根和布莱克测试了几种电气和机械刺激的组合, 报告说, 刺激之间的时间是至关重要的, 因为延迟联合机电刺激产生了最好的结果28。接下来, godier-fufurnémont 和合作者从新生大鼠心脏细胞中优化了工程心脏肌肉结构的机电刺激协议, 并首次实现了正力频率关系29。随后, 我们的研究小组报告说, 机电预处理细胞在体外增加了主要心脏标记物的表达, 并在体内产生了广泛的有益作用, 如心脏功能的改善或梗死血管密度的增加边境地区30。最近的出版物表明, 干细胞衍生心肌细胞的心脏组织在机电条件下达到了接近成人成人心脏结构和功能31的成熟水平.此外, 替代的三维刺激平台包括电活性支架, 为所附单元提供电气、机械和地形提示 32.此外, 机械变形 (细胞单层拉伸和压缩) 也可以通过模仿正常生理条件以及极端条件33的可拉伸电极引起。
因此, 其基本原理是, 基于生理条件的体外机电刺激可以增强细胞的心肌潜能。事实上, 这种刺激可以有利于进一步整合治疗细胞到心肌在临床情况下, 或增加组织成熟的药物筛选应用。
此外, 我们分离并鉴定了人类脂肪组织源性心脏祖细胞 (心脏 atdpc)34。这些细胞位于心外膜脂肪中。这些细胞在心肌梗死治疗中表现出有益的组织病理学和功能作用, 同时也保持心脏和内皮分化的潜力。30,35. 我们假设, 在生物物理刺激之后, 这些好处会增加。
因此, 我们为感兴趣的细胞群开发了一种装置和刺激机制, 并对其影响进行了调查。与以前的出版物36相比, 这种机电协议是以无菌和非侵入性的方式诱导主动细胞拉伸的新策略。这里报道的技术详细说明了用于细胞的电气、机械和机电刺激的装置和方法。
该装置可以独立或同时提供电和机械刺激。刺激是用一种非侵入性和无菌的新方法进行的, 该方法包括预灭菌的细胞支架、放置在标准培养板内的电极以及诱导机械和电力的平台 (图 1)。
该平台最多可容纳六个培养板, 由激光切割聚 (甲基丙烯酸甲酯) 和印刷电路板件组成。该平台原型依靠的组合单相可编程计算机控制的电刺激器, 印刷电路板的强大连接的电极, 和 6 10 毫米 x 10 毫米镍镀镍固定的磁铁放置附近的一面的培养板。也有一个铝棒与六个驱动磁铁 (同一模型) 放置在前面的另一侧的培养板, 并移动与线性伺服马达。电机由电机控制器驱动, 通过 rs-232 端口由商业软件操作 (见材料表)。通过用户界面和可编程刺激器, 可以对电强度、脉冲持续时间和频率、机械刺激频率、占空比、脉冲数、脉冲振幅 (磁体偏移) 进行编程,和斜坡。
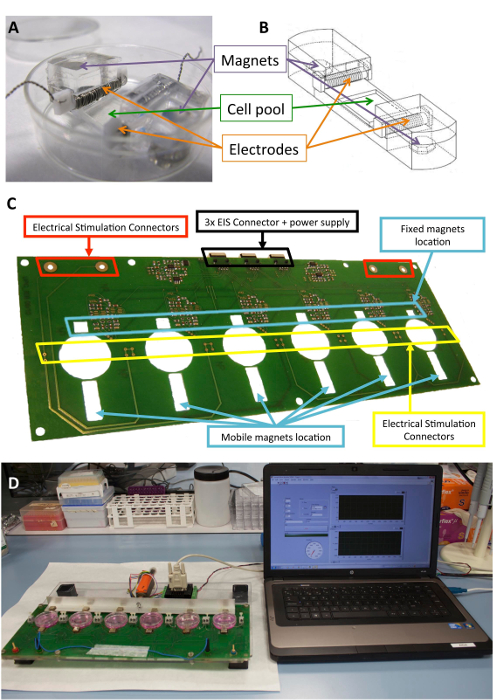
图 1: 机电刺激器.(a) 用于细胞调理的 pdms 构造。(b) 绘制 pdms 结构, 包括电极和磁铁。(c) 用于执行机电调理的印刷电路板 (平台) 的详细情况。该面板已从 llucia-valdeperas 等人30 人处进行了改装。(d) 机电刺激平台和用户界面 (计算机) 的图片。请点击这里查看此图的较大版本.
在两项国际专利中都充分介绍了刺激装置和机电调理方法, 即 wo-2013185818-a137和 wo-201725159-a138。
生物兼容型有机硅结构旨在为电池、电极和磁体提供结构支持, 此前已有 10、21 种。简单地说, 它们由聚二甲基硅氧烷 (pdms) 组成, 在室温下成型和固化, 杨氏模量为 1.3 mpa, 接近生理水平。该结构包含一个灵活区域 (10 毫米 x 10 毫米 x 2 毫米) 中的细胞培养池、两个固定电极的内部横向插槽和两个嵌入的6毫米 x2 毫米 x4 mm 镀镍的磁铁。电极是由0.2 毫米铂线扭曲周围的2毫米 x 3 毫米 x 12 毫米聚四氟乙烯 (ptfe) 核心酒吧 (21 厘米每个电极, 约23转), 并放置在对面的灵活区域, 以创建一个电场诱导电刺激。机械拉伸是通过在支撑中嵌入的磁体和放置在培养板旁边的外部磁体之间以及在移动的铝臂上的磁吸引来实现的。这样, 细胞支持可以在不打破无菌屏障的情况下扩展。这种方法适用于细胞单层, 但也可以适应三维结构。
此外, 使用规则衍射光栅 (1, 250 声), 可以在细胞播种的地方印上规则图案。由于其透明度和 0.5 mm 的厚度, 在明亮场和荧光显微镜下的 pdms 结构下培养的细胞是可能的直接可视化。在目前的情况下, pdms 培养池具有垂直的表面图案, 垂直于拉伸力, 以将细胞垂直地与电场对齐, 从而最大限度地减少整个细胞的电场梯度。
图 1显示了用于刺激的构造和设备的详细说明。pdms 结构和特性针对细胞拉伸进行了优化 (图 1a. b)。该刺激器的开发和验证是为了有效地应用所需的电和机械刺激的细胞连接到 pdms 结构。此过程包括通过软件界面确保良好的连接性和用户可操作性 (图 1C,D)。
协议部分介绍了使用此定制设备进行细胞刺激的过程。
Protocol
Representative Results
Discussion
机电刺激似乎是一个安全的选择, 准备细胞的敌对心脏环境, 并提高他们的心脏承诺。在这里, 描述的心脏祖细胞的协议增加了主要心脏标记的表达, 并被报道是有益的, 他们的下一个植入梗死小鼠心肌 30.一般来说, 机电刺激的心脏 atdpc 增加了与早期、结构和钙调节相关的基因表达, 而这在以前的电气或机械刺激中从未单独实现过。事实上, 机电刺激的心脏 atdpc 显示出一个更完整的…
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
作者要感谢 icrec 研究项目 (igtp, badalona) 和电子和生物医学仪器仪表组 (upc, 巴塞罗那) 的成员, 特别是 j. rocel-ferrer 教授。此外, 作者还承认 stem cels 转化医学杂志和 alphamed 出版社允许改编以前发表的数字 (llucia-valldeperas 等).30). 这一原型的开发和议定书的设计得到了欧洲联盟委员会第7个框架方案 (saf 2014-59892)、经济和竞争事务部 (saf—-第7个框架方案) 的支持。Marató, nmp3-sl-2009-29239)、fundacióla maratóde tv3 (080330, 201516, 201516) 和 fundación para la Prospectiva y la prospectivión en salud en españa (fipse; 06-00001396-15)。
Materials
| Stimulator | |||
| nickel plated neodymium magnets | Supermagnete | Q-10-10-05-N | |
| nickel-plated neodymium magnets | Supermagnete | Q-06-04-02-HN | |
| polydimethylsiloxane (PDMS) SYLGAR 184 Silicone Elastomer Kit | Dow Corning Corp | 184 | |
| ruled diffraction grating (1250 grooves/mm) | Newport | 05RG150-1250-2 | |
| Motor controller | Faulhaber | MCLM-3006-S | |
| Labview | National Instruments | ||
| Cell culture | |||
| phosphate-buffered saline (PBS) | Gibco | 70013-065 | |
| 0.05% trypsin-EDTA | Gibco | 25300-120 | |
| 35 mm cell culture dish | BD Falcon | 45353001 | |
| fetal bovine serum (FBS) | Gibco | 10270-106 | |
| L-Glutamine 200 mM, 100x | Gibco | 25030-024 | |
| Penicilina/Streptomicine, 10.000 U/mL | Gibco | 15140-122 | |
| Minimum essential medium eagle (alfa-MEM) | Sigma | M4526-24x500ML | |
| Protein & RNA analyses | |||
| protease inhibitor cocktail | Sigma | P8340 | |
| QIAzol Lysis Reagent | Qiagen | 79306 | |
| AllPrep RNA/Protein Kit | Qiagen | 50980404 | |
| Rneasy mini kit | Qiagen | 74104 | |
| iTaq Universal Probes One-Step Kit | Bio-Rad Laboratories | 172-5140 | |
| Random hexamers | Qiagen | 79236 | |
| TaqMan PreAmp MasterMix 2X | Applied Biosystems | 4391128 | |
| TaqMan Universal PCR MasterMix | Applied Biosystems | 4324018 | |
| Immunostaining | |||
| 10% formalin | Sigma | HT-501128-4L | |
| horse serum | Sigma | H1138 | |
| Triton X-100 | Sigma | X100-500ML | |
| Bovine Serum Albumina (BSA) | Sigma | A7906-100G | |
| PARAFILM | Sigma | P6543 | |
| 4′,6-diamidino-2-phenylindole (DAPI) | Sigma | D9542 | |
| Phalloidin Alexa 568 | Invitrogen | A12380 | |
| sodium azide | Sigma | S8032-100g | |
| Hoechst 33342 | Sigma | 14533 | |
| Connexin-43 rabbit primary antibody | Sigma | C6219 lot#061M4823 | |
| sarcomeric α-actinin mouse primary antibody | Sigma | A7811 lot#080M4864 | |
| GATA-4 goat primary antibody | R&D | AF2606 VAZ0515101 | |
| MEF2 rabbit primary antibody | Santa Cruz | sc-313 lot#E0611 | |
| SERCA2 goat primary antibody | Santa Cruz | sc-8095 lot#D2709 | |
| Cy3 secondary antibody | Jackson ImmunoResearch | 711-165-152 | |
| Cy3 secondary antibody | Jackson ImmunoResearch | 715-165-151 | |
| Cy3 secondary antibody | Jackson ImmunoResearch | 712-165-150 | |
| Cy2 secondary antibody | Jackson ImmunoResearch | 715-225-150 | |
| Cy2 secondary antibody | Jackson ImmunoResearch | 711-225-152 | |
| Cy2 secondary antibody | Jackson ImmunoResearch | 705-225-147 |
Referencias
- McDonough, P. M., Glembotski, C. C. Induction of atrial natriuretic factor and myosin light chain-2 gene expression in cultured ventricular myocytes by electrical stimulation of contraction. Journal of Biological Chemistry. 267, 11665-11668 (1992).
- Tandon, N., et al. Electrical stimulation systems for cardiac tissue engineering. Nature Protocols. 4, 155-173 (2009).
- Serena, E., et al. Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Experimental Cell Research. 315, 3611-3619 (2009).
- Tandon, N., et al. Optimization of electrical stimulation parameters for cardiac tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. 5, 115-125 (2011).
- Zhang, X., Wang, Q., Gablaski, B., Lucchesi, P., Zhao, Y. A microdevice for studying intercellular electromechanical transduction in adult cardiac myocytes. Lab on a Chip. 13, 3090-3097 (2013).
- Chan, Y. C., et al. Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells. Journal of Cardiovascular Translational Research. 6, 989-999 (2013).
- Pietronave, S., et al. Monophasic and biphasic electrical stimulation induces a precardiac differentiation in progenitor cells isolated from human heart. Stem Cells and Development. 23, 888-898 (2014).
- Pavesi, A., et al. Electrical conditioning of adipose-derived stem cells in a multi-chamber culture platform. Biotechnology and Bioengineering. 111, 1452-1463 (2014).
- Baumgartner, S., et al. Electrophysiological and morphological maturation of murine fetal cardiomyocytes during electrical stimulation in vitro. Journal of Cardiovascular Pharmacology and Therapeutics. 20, 104-112 (2015).
- Llucià-Valldeperas, A., et al. Electrical stimulation of cardiac adipose tissue-derived progenitor cells modulates cell phenotype and genetic machinery. Journal of Tissue Engineering and Regenerative Medicine. 9 (11), 76-83 (2015).
- Llucià-Valldeperas, A., et al. Physiological conditioning by electric field stimulation promotes cardiomyogenic gene expression in human cardiomyocyte progenitor cells. Stem Cell Research and Therapy. 5, 93 (2014).
- Radisic, M., et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings of the National Academy of Sciences of the United States of America. 101 (52), 18129-18134 (2004).
- Fink, C., et al. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB Journal. 14, 669-679 (2000).
- Zimmermann, W. H., et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nature Medicine. 12 (4), 452-458 (2006).
- Birla, R. K., Huang, Y. C., Dennis, R. G. Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Engineering. 13, 2239-2248 (2007).
- Salameh, A., et al. Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circulation Research. 106, 1592-1602 (2010).
- Galie, P. A., Stegemann, J. P. Simultaneous application of interstitial flow and cyclic mechanical strain to a three-dimensional cell-seeded hydrogel. Tissue Engineering Part C: Methods. 17 (5), 527-536 (2011).
- Leychenko, A., Konorev, E., Jijiwa, M., Matter, M. L. Stretch-induced hypertrophy activates NFkB-mediated VEGF secretion in adult cardiomyocytes. PLoS One. 6, 29055 (2011).
- Tulloch, N. L., et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circulation Research. 109, 47-59 (2011).
- Mihic, A., et al. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials. 35, 2798-2808 (2014).
- Llucià-Valldeperas, A., et al. Unravelling the effects of mechanical physiological conditioning on cardiac adipose tissue-derived progenitor cells in vitro and in silico. Scientific Reports. 8, 499 (2018).
- Stoppel, W. L., Kaplan, D. L., Black, L. D. Electrical and mechanical stimulation of cardiac cells and tissue constructs. Advanced Drug Delivery Reviews. 96, 135-155 (2016).
- Nunes, S. S., et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nature Methods. 10, 781-787 (2013).
- Barash, Y., et al. Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering. Tissue Engineering Part C: Methods. 16, 1417-1426 (2010).
- Maidhof, R., et al. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. Journal of Tissue Engineering and Regenerative Medicine. 6, 12-23 (2012).
- Feng, Z., et al. An electro-tensile bioreactor for 3-D culturing of cardiomyocytes. A bioreactor system that simulates the myocardium’s electrical and mechanical response in vivo. IEEE Engineering in Medicine and Biology Magazine. 24 (4), 73-79 (2005).
- Wang, B., et al. Myocardial scaffold-based cardiac tissue engineering: application of coordinated mechanical and electrical stimulations. Langmuir. 29 (35), 11109-11117 (2013).
- Morgan, K. Y., Black, L. D. Mimicking isovolumic contraction with combined electromechanical stimulation improves the development of engineered cardiac constructs. Tissue Engineering Part A. 20 (11-12), 1654-1667 (2014).
- Godier-Furnémont, A. F., et al. Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation. Biomaterials. 60, 82-91 (2015).
- Llucià-Valldeperas, A., et al. Electromechanical Conditioning of Adult Progenitor Cells Improves Recovery of Cardiac Function After Myocardial Infarction. Stem Cell Translational Medicine. 6 (3), 970-981 (2017).
- Ronaldson-Bouchard, K., et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 556 (7700), 239-243 (2018).
- Gelmi, A., et al. Direct Mechanical Stimulation of Stem Cells: A Beating Electromechanically Active Scaffold for Cardiac Tissue Engineering. Advanced Healthcare Materials. 5 (12), 1471-1480 (2016).
- Poulin, A., et al. An ultra-fast mechanically active cell culture substrate. Scientific Reports. 8 (1), 9895 (2018).
- Bayes-Genis, A., et al. Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. Journal of Molecular and Cellular Cardiology. 49 (5), 771-780 (2010).
- Bagó, J. R., et al. Bioluminescence imaging of cardiomyogenic and vascular differentiation of cardiac and subcutaneous adipose tissue-derived progenitor cells in fibrin patches in a myocardium infarct model. International Journal of Cardiology. 169, 288-295 (2013).
- Zimmermann, W. H., et al. Tissue engineering of a differentiated cardiac muscle construct. Circulation Research. 90 (2), 223-230 (2002).
- Rosell Ferrer, F. X., Sánchez Terrones, B., Bragós Bardia, R., Bayés Genís, A., Llucià Valldeperas, A. Methods and devices for mechanical and electrical stimulation of stem cell monolayer and 3d cultures for tissue engineering applications. Spanish patent. , (2013).
- Bayés Genís, A., Llucià Valldeperas, A., Soler Botija, C., Bragós Bardia, R., Rosell Ferrer, F. X. Method for Conditioning Stem Cells. Spanish patent. , (2017).
- Roura, S., Gálvez-Montón, C., Bayes-Genis, A. Myocardial healing using cardiac fat. Expert Review of Cardiovascular Therapy. 16 (4), 305-311 (2018).
- Zhang, Y. M., Hartzell, C., Narlow, M., Dudley, S. C. Stem cell-derived cardiomyocytes demonstrate arrhythmic potential. Circulation. 106 (10), 1294-1299 (2002).
- Liu, J., Fu, J. D., Siu, C. W., Li, R. A. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 25 (12), 3038-3044 (2007).
- Wipff, P. J., et al. The covalent attachment of adhesion molecules to silicone membranes for cell stretching applications. Biomaterials. 30 (9), 1781-1789 (2009).
- Kim, C. iPSC technology–Powerful hand for disease modeling and therapeutic screen. Biochemistry and Molecular Biology Reports. 48 (5), 256-265 (2015).
- Ronaldson-Bouchard, K., Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell. 22 (3), 310-324 (2018).
- Bruyneel, A. A., McKeithan, W. L., Feyen, D. A., Mercola, M. Will iPSC-cardiomyocytes revolutionize the discovery of drugs for heart disease. Current Opinion inPharmacology. 42, 55-61 (2018).
- Farley, A., Johnstone, C., Hendry, C., McLafferty, E. Nervous system: part 1. Nursing Standard. 28 (31), 46-51 (2014).
- Brotto, M., Bonewald, L. Bone and muscle: Interactions beyond mechanical. Bone. 80, 109-114 (2015).
- Park, S. J., et al. Neurogenesis Is Induced by Electrical Stimulation of Human Mesenchymal Stem Cells Co-Cultured With Mature Neuronal Cells. Macromolecular Bioscience. 15 (11), 1586-1594 (2015).
- Vianney, J. M., Miller, D. A., Spitsbergen, J. M. Effects of acetylcholine and electrical stimulation on glial cell line-derived neurotrophic factor production in skeletal muscle cells. Brain Research. 1588, 47-54 (2014).
- Shima, A., Morimoto, Y., Sweeney, H. L., Takeuchi, S. Three-dimensional contractile muscle tissue consisting of human skeletal myocyte cell line. Experimental Cell Research. 370 (1), 168-173 (2018).

