通过颈内动脉注射癌细胞模拟脑转移
Summary
脑转移是癌症患者严重发病和死亡的原因。大多数脑转移小鼠模型因全身转移而复杂化,混淆死亡率和治疗干预结果的分析。这里介绍的是颈动脉内注射癌细胞的方案,该方案产生一致的颅内肿瘤和最小的全身性肿瘤。
Abstract
脑转移是癌症患者严重发病和死亡的原因。转移性疾病的关键方面,例如复杂的神经微环境和基质细胞相互作用,不能完全通过 体外 测定复制;因此,动物模型对于研究和理解治疗干预的效果至关重要。然而,大多数脑肿瘤异种移植方法在时间框架和肿瘤负荷方面不能一致地产生脑转移。通过心内注射癌细胞产生的脑转移模型可导致意外的颅外肿瘤负荷,并导致非脑转移发病率和死亡率。虽然颅内注射癌细胞可以限制颅外肿瘤的形成,但它有几个注意事项,例如注射的细胞经常在注射部位形成单一的肿瘤肿块,高软脑膜受累,以及在针刺穿透过程中脑血管系统受损。该协议描述了由颈内动脉注射产生的脑转移的小鼠模型。该方法在没有其他器官参与的情况下持续产生颅内肿瘤,从而能够评估脑转移的治疗剂。
Introduction
脑转移是一种普遍的恶性肿瘤,预后非常差1,2。脑转移患者的护理标准是多模式的,包括神经外科、全脑放疗和/或立体定向放射外科手术,具体取决于患者的一般健康状况、颅外疾病负担以及脑部肿瘤的数量和位置3,4。最多 3 个颅内病变的患者适合手术切除或立体定向放射外科手术,而建议有多个病变的患者进行全脑放疗,以避免手术相关感染和水肿的风险5.然而,全脑放疗会对放射敏感的大脑结构造成损害,导致生活质量下降6。
全身治疗是治疗多发病变患者的非侵入性替代和合乎逻辑的方法7。然而,由于长期以来认为全身治疗疗效不佳,因为通过血流被动递送细胞毒性药物无法在没有不安全毒性风险的情况下达到大脑中的治疗水平,因此较少考虑8。随着最近美国食品和药物管理局(FDA)批准的全身治疗(图卡替尼联合曲妥珠单抗和卡培他滨用于转移性HER2+乳腺癌脑转移)9,10,11,12以及治疗指南的更新,包括考虑脑转移患者的全身治疗选择,这种范式开始改变13,14。
在这种情况下,分子靶向治疗,免疫疗法和替代药物递送系统(例如靶向纳米药物载体)领域的发展可以潜在地克服脑转移治疗的挑战15,16,17,18。此外,还正在研究通过脑肿瘤屏障透化来改善药物递送的化学和机械方法19,20。为了研究和优化这些方法以适应目的,使用临床前模型至关重要,这些模型不仅反映了脑转移的复杂生理学,而且还允许对颅内药物反应进行客观分析。
从广义上讲,目前模拟体内脑转移的方法涉及心内(左心室),静脉内(通常是尾静脉),颅内或颈内(颈总动脉)注射小鼠的癌细胞21,22,23,24,25,26,27.除了肿瘤植入策略外,通过去除肿瘤抑制基因或激活癌基因触发肿瘤形成的基因工程小鼠模型可用于肿瘤建模。然而,据报道,只有少数基因工程小鼠模型产生继发性肿瘤,甚至更少可靠地产生脑转移瘤28,29,30。
植入方法,如心内(左心室)和静脉注射(通常是尾静脉)模仿癌症的全身播散。这些模型通常在多个器官(例如,脑、肺、肝、肾、脾脏)中产生病变,这取决于在循环“首次通过”期间捕获大多数肿瘤细胞的毛细血管床31。然而,不一致的脑植入率将需要更多的动物才能达到所需统计功效的样本量。通过这些心内和静脉注射方法 最终 在大脑中建立的肿瘤细胞数量是可变的。因此,脑转移肿瘤负荷可能因动物而异,进展的差异可能使标准化实验时间表和结果解释成为一项挑战。颅外肿瘤负荷可导致非脑转移死亡,使这些模型不适合评估颅内疗效。已经使用人工克隆选择过程建立了脑-热带细胞系以减少颅外建立,但摄取速率不一致,并且克隆选择过程可以降低通常在人类肿瘤中发现的异质性32。
脑特异性植入方法,如颅内和颈内注射,可实现更一致和有效的脑转移建模。在颅内方法33中,癌细胞通常被注射到额叶大脑皮层中,其产生快速且可重复的肿瘤生长,全身受累低。虽然该手术耐受性良好,死亡率低33,但需要注意的是,这是一种相对粗糙的方法,可迅速在大脑中引入(局部)细胞推注,并且不能模拟早期脑转移发病机制。针头会破坏脑组织脉管系统,然后引起局部炎症5,34。根据经验,在拔针过程中,肿瘤细胞注射有反流的趋势,导致软脑膜受累。或者,颈内方法将细胞输送到颈总动脉中,脑微脉管系统作为遇到的第一个毛细血管床,模拟循环、外渗和定植的存活率24。与其他人一致25,我们使用这种方法的经验发现,由于癌细胞通过颈外动脉 无意 中递送到这些组织中的毛细血管床,它可能导致面部肿瘤(未发表的数据)。通过在颈总动脉注射之前首先结扎颈外动脉可以预防面部肿瘤(图1)。在本文的其余部分,这种方法被称为“颈内动脉注射”。根据经验,颈内动脉注射方法始终如一地产生脑转移,全身性事件很少,并且已成功生成不同原发性癌症(例如黑色素瘤、乳腺癌和肺癌)的脑转移模型(图 1)。缺点是它在技术上具有挑战性、耗时、侵入性强,并且需要仔细优化细胞数量和监测时间表。总之,颅内和颈内动脉注射方法都产生了适合评估对脑肿瘤相关生存益处的治疗影响的小鼠模型。
该协议描述了颈内动脉注射方法,以产生几乎没有全身参与的脑转移小鼠模型,因此适用于药物分布和实验疗法疗效的临床前评估。
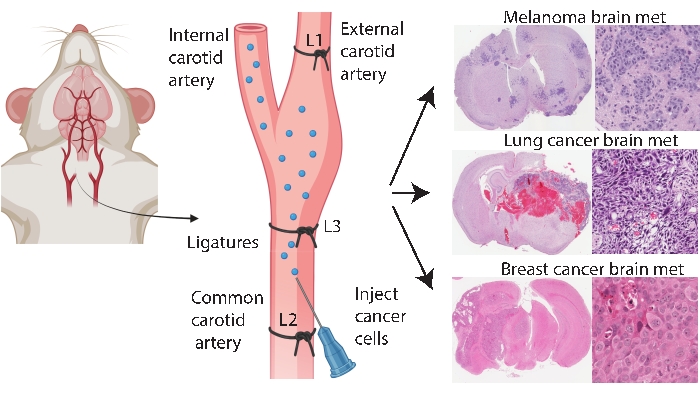
图1:脑转移的颈内动脉注射方案示意图。 颈内动脉注射与颈外动脉结扎可以可靠地产生来自各种原发性癌症的脑转移模型。在该协议中,三个结扎放置在颈动脉上(图中注释为L1-L3)。 请点击此处查看此图的大图。
Protocol
Representative Results
Discussion
脑转移是癌细胞从原发部位扩散到大脑的复杂过程。有不同的动物模型可以反映这个多步骤过程的某些阶段,并且设计临床前转移研究有生理和实际考虑41,42。大多数已发表的研究调查使用纳米药物进行脑转移治疗的研究都使用了心内43,44和颅内45,46,47,48,<sup …
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
这项研究由澳大利亚国家健康与医学研究委员会(NHMRC)资助,批准号为APP1162560。ML由昆士兰大学研究生研究奖学金资助。我们要感谢所有协助畜牧业和动物 体内 成像的人。我们感谢皇家布里斯班妇女医院为这项研究捐赠等分试样的锆。
Materials
| 100µm cell strainer | Corning | CLS431752 | |
| 30G Microlance needle | BD | 23748 | |
| 31G Ultra-Fine II insulin syringe | BD | 326103 | |
| Angled forceps | Proscitech | T67A-SS | Fine pointed, angled without serrations, 18mm tip, length 128 mm |
| Animal heat mat | |||
| Antibiotic and antimycotic | ThermoFisher Scientific | 15240062 | |
| Autoclave bags | |||
| BT-474 (HTB-20) breast cancer cell line | ATCC | HTB-20 | |
| Buprenorphine (TEMGESIC) | |||
| Countess cell counter | ThermoFisher Scientific | C10227 | |
| Diet-76A | ClearH2O | 72-07-5022 | |
| Dissection microscope | |||
| Ear puncher | |||
| Electric clippers | |||
| Fine angled forceps | Proscitech | DEF11063-07 | Angled 45°, Tip smooth, Tip width: 0.4 mm, Tip dimension: 0.4 x 0.3 mm, length 9cm |
| Fine tubing for cannula, Tubing OD (in) 1/32, Tubing ID (in) 1/100in | Cole Parmer | EW-06419-00 | |
| Foetal bovine serum | ThermoFisher Scientific | 26140079 | |
| Hank's Balanced Salt Solution without calcium and magnesium | ThermoFisher Scientific | 14170120 | |
| Hydrogel | ClearH2O | 70-01-5022 | |
| Isoflurane | |||
| Kimwipes Low lint disposable wipers | Kimberly Clark- Kimwipes | Z188964 | |
| Mashed mouse chow | |||
| Meloxicam (METACAM) | |||
| Nose cone | Fashioned out of a microfuge tube | ||
| PAA ocular lubricant (Carbomer 2mg/g) | Bausch and lomb | ||
| Povidone-iodine solution | Betadine | 2505692 | |
| PPE (glove, mask, gown, hairnet) | |||
| Retractors | Kent Scientific | SURGI-5001 | |
| RPMI 1640 Media | ThermoFisher Scientific | 11875093 | |
| Silk suture 13mm 5-0, P3, 45cm | Ethicon | JJ-640G | |
| Sterile normal saline | ThermoFisher Scientific | TM4469 | |
| Sticky tape | |||
| Surgical board | A chopping board wrapped with autoclavable bag. | ||
| Surgical scissors | Proscitech | T104 | Tip Dimensions (LxD): 38x7mm, Length 115mm |
| Suture forcep/ Curved Brophy forceps | Proscitech | T113C | Curved, Rounded narrow 2 mm tip, with serrations, length 165 mm |
| Suture needle holder (Olsen Hegar needle holder) | Proscitech | TC1322-180 | length 190 mm, ratchet clamp |
| Syringe driver with foot pedal/ UMP3 Ultra micro pump | World Precision Instruments | UMP3-3 | |
| T75 tissue culture flask | ThermoFisher Scientific | 156499 | |
| Thread | |||
| Trigene II surface disinfectant | Ceva | ||
| Trypan Blue and Cell Counting Chamber Slides | ThermoFisher Scientific | C10228 | |
| TrypLE Express dissociating medium | ThermoFisher Scientific | 12605010 |
Referencias
- Nayak, L., Lee, E. Q., Wen, P. Y. Epidemiology of brain metastases. Current Oncology Reports. 14 (1), 48-54 (2012).
- . Australian Institute of Health and Welfare. Cancer in Australia. , (2017).
- Maher, E. A., Mietz, J., Arteaga, C. L., DePinho, R. A., Mohla, S. Brain metastasis: opportunities in basic and translational research. Investigación sobre el cáncer. 69 (15), 6015-6020 (2009).
- Lin, N. U. Breast cancer brain metastases: new directions in systemic therapy. Ecancermedicalscience. 7, (2013).
- Zimmer, A. S., Van Swearingen, A. E. D., Anders, C. K. HER2-positive breast cancer brain metastasis: A new and exciting landscape. Cancer Reports. 5 (4), (2020).
- Brown, P. D., et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncology. 18 (8), 1049-1060 (2017).
- Murrell, J., Board, R. The use of systemic therapies for the treatment of brain metastases in metastatic melanoma: Opportunities and unanswered questions. Cancer Treatment Reviews. 39 (8), 833-838 (2013).
- Stemmler, H. J., et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 18 (1), 23-28 (2007).
- Venur, V. A., Leone, J. P. Targeted therapies for brain metastases from breast cancer. International Journal of Molecular Sciences. 17 (9), 1543 (2016).
- Murthy, R., et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. The Lancet Oncology. 19 (7), 880-888 (2018).
- Murthy, R. K., et al. trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. New England Journal of Medicine. 382 (7), 597-609 (2019).
- Shah, M., et al. FDA approval summary: Tucatinib for the treatment of patients with advanced or metastatic HER2-positive breast cancer. Clinical Cancer Research. 27 (5), 1220-1226 (2021).
- Vogelbaum, M. A., et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. Journal of Clinical Oncology. 40 (5), 492-516 (2021).
- Ramakrishna, N., et al. Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. Journal of Clinical Oncology. 10, (2022).
- Li, J., et al. A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood-brain barrier targeting brain metastases of breast cancer. ACS Nano. 8 (10), 9925-9940 (2014).
- Mittapalli, R. K., et al. Paclitaxel-hyaluronic nanoconjugates prolong overall survival in a preclinical brain metastases of breast cancer model. Molecular Cancer Therapeutics. 12 (11), 2389-2399 (2013).
- Hamilton, A. M., et al. Nanoparticles coated with the tumor-penetrating peptide iRGD reduce experimental breast cancer metastasis in the brain. Journal of Molecular Medicine. 93 (9), 991-1001 (2015).
- Patil, R., et al. MRI virtual biopsy and treatment of brain metastatic tumors with targeted nanobioconjugates: nanoclinic in the brain. ACS Nano. 9 (5), 5594-5608 (2015).
- Brighi, C., et al. MR-guided focused ultrasound increases antibody delivery to non-enhancing high-grade glioma. Neuro-Oncology Advances. 2 (1), (2020).
- Inamura, T., Black, K. L. Bradykinin selectively opens blood-tumor barrier in experimental brain tumors. Journal of Cerebral Blood Flow & Metabolism. 14 (5), 862-870 (1994).
- Priego, N., et al. Abstract 2746: Stat3 labels a subpopulation of reactive astrocytes required for brain metastasis. Investigación sobre el cáncer. 79, 2746 (2019).
- Wyatt, E. A., Davis, M. E. Method of establishing breast cancer brain metastases affects brain uptake and efficacy of targeted, therapeutic nanoparticles. Bioengineering & Translational Medicine. 4 (1), 30-37 (2018).
- Nakayama, J., et al. The in vivo selection method in breast cancer metastasis. International Journal of Molecular Sciences. 22 (4), 1886 (2021).
- Zhang, C., Lowery, F. J., Yu, D. Intracarotid cancer cell injection to produce mouse models of brain metastasis. Journal of Visualized Experiments. 120, 55085 (2017).
- Liu, Z., et al. Improving orthotopic mouse models of patient-derived breast cancer brain metastases by a modified intracarotid injection method. Scientific Reports. 9 (1), 622 (2019).
- Bos, P. D., et al. Genes that mediate breast cancer metastasis to the brain. Nature. 459, 1005-1009 (2009).
- Hu, X., Villodre, E. S., Woodward, W. A., Debeb, B. G. Modeling brain metastasis via tail-vein injection of inflammatory breast cancer cells. Journal of Visualized Experiments. 168, (2021).
- Cho, J. H., et al. AKT1 activation promotes development of melanoma metastases. Cell Reports. 13 (5), 898-905 (2015).
- Meuwissen, R., et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 4 (3), 181-189 (2003).
- Kato, M., et al. Transgenic mouse model for skin malignant melanoma. Oncogene. 17 (14), 1885-1888 (1998).
- Khanna, C., Hunter, K. Modeling metastasis in vivo. Carcinogenesis. 26 (3), 513-523 (2005).
- Sulaiman, A., Wang, L. Bridging the divide: preclinical research discrepancies between triple-negative breast cancer cell lines and patient tumors. Oncotarget. 8 (68), 113269-113281 (2017).
- Pierce, A. M., Keating, A. K. Creating anatomically accurate and reproducible intracranial xenografts of human brain tumors. Journal of Visualized Experiments. 91, 52017 (2014).
- Geisler, J. A., et al. Modeling brain metastases through intracranial injection and magnetic resonance imaging. Journal of Visualized Experiments. 160, (2020).
- Reid, Y., Storts, D., Riss, T., Minor, L., et al. . in Assay Guidance Manual. eds Markossian, S. et al.) Eli Lilly & Company and the National Center for Advancing Translational Sciences. , (2004).
- Janowicz, P. W., et al. Understanding nanomedicine treatment in an aggressive spontaneous brain cancer model at the stage of early blood brain barrier disruption. Biomaterials. , 283 (2022).
- Houston, Z. H., et al. Understanding the Uptake of Nanomedicines at Different Stages of Brain Cancer Using a Modular Nanocarrier Platform and Precision Bispecific Antibodies. ACS Cent Sci. 6 (5), 727-738 (2020).
- Matsumura, Y., Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Investigación sobre el cáncer. 46, 6387-6392 (1986).
- Clemons, T. D., et al. Distinction between active and passive targeting of nanoparticles dictate their overall therapeutic efficacy. Langmuir. 34 (50), 15343-15349 (2018).
- Wu, J., et al. Transcardiac perfusion of the mouse for brain tissue dissection and fixation. Bio-Protocol. 11 (5), (2021).
- Masmudi-Martín, M., et al. Brain metastasis models: What should we aim to achieve better treatments. Advanced Drug Delivery Reviews. 169 (20), 79-99 (2021).
- Carney, C. P., et al. Harnessing nanomedicine for enhanced immunotherapy for breast cancer brain metastases. Drug Delivery and Translational Research. 11 (6), 2344-2370 (2021).
- Hamilton, A. M., et al. Nanoparticles coated with the tumor-penetrating peptide iRGD reduce experimental breast cancer metastasis in the brain. Journal of Molecular Medicine. 93 (9), 991-1001 (2015).
- Bao, Y., et al. Synergistic chemotherapy for breast cancer and breast cancer brain metastases via paclitaxel-loaded oleanolic acid nanoparticles. Molecular Pharmaceutics. 17 (4), 1343-1351 (2020).
- Kotb, S., et al. Gadolinium-based nanoparticles and radiation therapy for multiple brain melanoma metastases: Proof of concept before phase I trial. Theranostics. 6 (3), 418-427 (2016).
- Zhang, T., et al. Multitargeted nanoparticles deliver synergistic drugs across the blood-brain barrier to brain metastases of triple negative breast cancer cells and tumor-associated macrophages. Advanced Healthcare Materials. 8 (18), 1900543 (2019).
- He, C., et al. Blood-brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. Journal of Controlled Release. 246, 98-109 (2017).
- Wang, X., et al. Enhanced anti-brain metastasis from non-small cell lung cancer of osimertinib and doxorubicin co-delivery targeted nanocarrier. International Journal of Nanomedicine. 15, 5491-5501 (2020).
- Gries, M., et al. Multiscale selectivity and in vivo biodistribution of NRP-1-targeted theranostic AGuIX nanoparticles for PDT of glioblastoma. International Journal of Nanomedicine. 15, 8739-8758 (2020).

