Developing and Testing Methylated Nano-Structured Dipeptides that Inhibit Src Kinase Activity In Vitro for Anti-Cancer Applications
Summary
Presented here is a protocol for designing and testing dipeptides that can inhibit Src kinase enzyme activity using acellular and cellular assays for anti-cancer applications. The peptides formulated here (W-RCH3, WCH3-RCH3, and W-R(CH3)2) inhibited Src kinase with IC50 values of 510 nM, 916 nM, and 1 µM, respectively.
Abstract
Here, with the aim of developing a novel anti-cancer treatment, seven dipeptides were designed that contained methylated tryptophan and/or methylated arginine and were produced using Fmoc solid-phase peptide synthesis. Overexpression of the Src tyrosine kinase enzyme has been implicated in the development of different cancers. Dipeptides containing unnatural amino acids such as methylated arginine (RCH3), dimethylated arginine (R(CH3)2), and/or methylated tryptophan (WCH3) residues have earlier been shown to inhibit Src kinase. In this study, three such dipeptides, W-RCH3, WCH3-RCH3, and W-R(CH3)2, were tested using acellular assays and were found to have IC50 values (the concentration at which 50% inhibition occurs) of 510 nM, 916 nM, and 1 µM, respectively. These values were comparable to those obtained for cyclic penta- to nano-W-R peptides ([W-R]5-[W-R]9) synthesized in previous studies. However, the unmethylated versions of the dipeptides did not show any inhibitory activity against Src kinase. All of these dipeptides (50 µM) did not show any cytotoxicity after incubation up to 72 h with three different cancer cell lines, including leukemia (CCRF-CEM), breast adenocarcinoma (MDA-MB-231), and ovarian adenocarcinoma (SK-OV-3) cell lines, indicating the limited permeability of the peptides through the cell membrane. Therefore, further study is needed to improve the permeability of these peptides for anticancer applications, such as by using a peptide carrier or additional peptide functionalization. In summary, this study provides a protocol to synthesize and test peptides that inhibit Src kinase activity, and thus possess promising anticancer ability, as demonstrated using acellular and cellular assays.
Introduction
Cancer is caused by the abnormal growth of normal cells and is one of the most lethal diseases around the world. These abnormal cells spread to different organs in the body by a process called metastasis. The most common form of cancer is breast cancer, which occurred in 2.26 million people in 2020. Moreover, there were around 1.80 million deaths due to lung cancer in 20201. According to the World Health Organization, around 10 million people died from cancer in 20202. Cancer cells differ from normal cells in that they overexpress certain enzymes, such as protein tyrosine kinases (PTKs). The National Cancer Institute defines kinases as enzymes able to phosphorylate other proteins or sugars3. Knowledge of the regulatory function of kinases can facilitate the design of effective anticancer drugs. For example, PTKs catalyze the phosphorylation of other proteins or sugars, and as a consequence, ATP is converted to ADP by the loss of a phosphate group. A total of 80% of oncogenes and protooncogenes encode PTKs4. Src kinases are a family of non-receptor tyrosine kinases, including Lck, Fyn, Hck, Blk, Yes, and Yrk, that are overexpressed in cancer cells, especially in breast cancer5,6. Src tyrosine kinases are associated with mitogenesis, differentiation, T-cell activation, and cell transformation. Src helps cancer cell invasion and metastasis due to its ability to reduce cancer cell adhesion. There are five different domains in Src kinase, ordered from the N- to C-terminals as: fatty acid domain, Src homology 3 domain (SH3), Src homology 2 domain (SH2), tyrosine kinase domain (SH1), and C-terminal regulatory domain7.
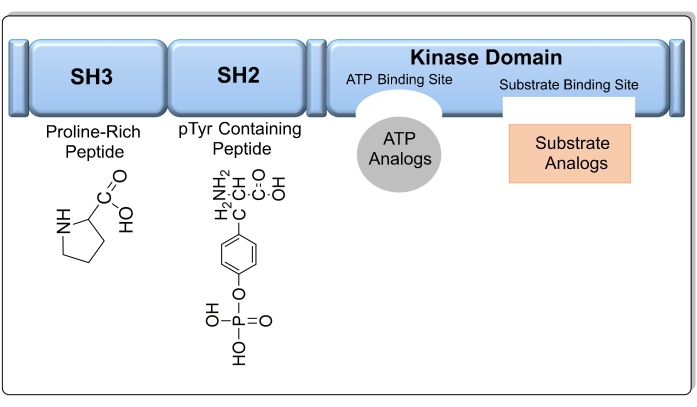
Figure 1: The target domains in the Src kinase enzyme, including a SH3 domain, SH2 domain, kinase domain (SH1), and a short C-terminal regulatory segment. Please click here to view a larger version of this figure.
The kinase domain SH1 is most commonly targeted when designing Src kinase inhibitors, as it contains two conserved sites for ATP and substrate binding (Figure 1). If the amino acid sequence of the kinase domain is known, the substrate can also be used as a target to design a compound that mimics substrate binding to Src kinase8. In addition, other sites such as the SH3 and SH2 domains can be used as targets. Compared to other chemotherapy agents, kinase inhibitors exhibit less toxicity and higher efficacy9. As of September 2021, there are 73 small molecules that act as kinase inhibitors that have been approved by the FDA10. Imatinib is an example of an anticancer drug that selectively inhibits the activity of tyrosine kinase; however, some patients are resistant to the drug due to the appearance of a point mutation in the kinase domain11. AstraZeneca released Saracatinib, which is a drug that inhibits the Src family of tyrosine kinases with an IC50 value (the concentration at which 50% inhibition occurs) of 2.7 nM, but it was discounted in phase 2 trials12. Of the 52 PTK inhibitors approved by the US FDA as of the beginning of 202013, only 28 target receptor PTKs, 11 block the non-receptor PTK, 11 inhibit protein-serine/threonine protein kinases, and two block MEK1/213. The increasing research interest in oncology will continue to fuel the discovery of kinase inhibitors as potential anti-cancer drugs. However, only 50 out of 500 protein kinases have been targeted for treatment thus far; therefore, a greater number of kinases are expected to be studied for drug development in the near future14. In addition, there is a need to discover kinase inhibitors to explore as yet unidentified kinase mutations that lead to cancer.
Thus, this study aimed to develop peptides that could be used as inhibitors for the Src family and target the ATP binding site due to its ability to serve as a conserved site between different kinases. To this end, a series of dipeptides containing methylated tryptophan and/or methylated arginine were synthesized and tested for their synergistic ability to inhibit Src kinase. The indole ring of tryptophan mimics the adenine of ATP and competes with ATP from binding to the ATP-binding site. In addition, the methylated arginine in the ligand competes for the SH3 domain of Src. Researchers showed that a polypeptide containing demethylated arginine inhibits the SH3 domain, possibly due to a specific conserved sequence on the SH3 binding motif (i.e., PXXP), which has a binding affinity to a ligand containing two to three Arg residues in the N-terminal or one to two Arg residues on the C-terminal of the ligands15,16,17. The guanidino group of Arg binds to the conserved Asp-99 residue of the SH3 domain18,19, while the remaining portion of the Arg binds to the conserved Trp-118 of the enzyme, as confirmed from NMR analysis and the crystal structures of several SH3 domains19. Here, a protocol for the synthesis of seven methylated dipeptides and testing their inhibition ability against Src kinase is presented. Further, the ability of these peptides to kill several cancer cell lines in vitro was examined.
Protocol
Representative Results
Discussion
The peptides fabricated and tested here for the inhibition of Src kinase and consequent killing of cancer cells contained methylated tryptophan and/or methylated arginine, which are unnatural amino acids. Formation of the white precipitate upon adding diethyl ether is a critical step in the synthesis of these peptides. However, not all synthetic peptides can form a precipitate; therefore, even when a precipitate is not formed, successful peptide synthesis can be confirmed by the determination of the desired mass using li…
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We would like to thank the Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU), Jeddah, Saudi Arabia, who has funded this project under grant no. (G: 031-130-1443).
Materials
| 1,4-Dithiothreitol | Sigma-Aldrich | 10708984001 | Peptide synthesis |
| Aldrich fritted filter funnel for solid-phase synthesis | Sigma-Aldrich | Z283304 | Peptide synthesis vessel |
| Alexa594 Tracer | Bell Brook Labs, Madison, WI | 3013 | |
| Anisole | Sigma-Aldrich | 8014520500 | |
| CellTiter 96 AQueous One Solution Cell Proliferation Assay reagents | Promega | G3582 | Cell proliferation assay (MTS reagent) |
| Dichloromethane, 99.9%, Extra Dry | Fishersci | AC326850025 | |
| Fmoc-ADMA(Pbf)-OH | Sigma-Aldrich | 8521070001 | |
| Fmoc-Arg(Me,Pbf)-OH | Sigma-Aldrich | 8521050001 | |
| Fmoc-Arg(Pbf)-OH | Sigma-Aldrich | 8520670025 | |
| Fmoc-Trp(Boc)-OH | Sigma-Aldrich | 47561-25G-F | |
| HPLC C18 column | Shimadzu (RP-HPLC system) | water/acetonitrile gradient | |
| IRDye QC-1 quencher | Bell Brook Labs, Madison, WI | 3013 | |
| Microplate reader SpectraMax M2e | Molecular devices | ||
| Microsoft Excel | Microsoft | spreadsheet software | |
| N,N-Diisopropylethylamine (DIPEA) | Sigma-Aldrich | 496219 | |
| N,N-Dimethylformamide, anhydrous, 99.8% | Fishersci | AA43997M1 | |
| Piperidine 20% | Sigma-Aldrich | 80645 | |
| Rink Amide resin (100-200 mesh) | Sigma-Aldrich | 8550010025 | |
| Thioanisole | Sigma-Aldrich | 92358 | |
| Transcreener ADP2 FI Assay | Bell Brook Labs, Madison, WI | 3013 | c-Src kinase activity assay kit |
Referencias
- Sung, H., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 71 (3), 209-249 (2021).
- Cancer. World Health Organization Available from: https://www.who.int/news-room/fact-sheets/detail/cancer#:~:text=Cancer%20a%20leading%20cause (2022)
- Negi, P., Cheke, R. S., Patil, V. M. Recent advances in pharmacological diversification of Src family kinase inhibitors. Egyptian Journal of Medical Human Genetics. 22 (1), 52 (2021).
- Huang, X. L., et al. Role of receptor tyrosine kinases mediated signal transduction pathways in tumor growth and angiogenesis-New insight and futuristic vision. International Journal of Biological Macromolecules. 180, 739-752 (2021).
- Mohammed, S., et al. Sublethal doxorubicin promotes migration and invasion of breast cancer cells: role of Src Family non-receptor tyrosine kinases. Breast Cancer Research. 23 (1), 76 (2021).
- Biscardi, J. S., Ishizawar, R. C., Silva, C. M., Parsons, S. J. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Research. 2 (3), 1-8 (2000).
- Ortiz, M. A., et al. Src family kinases, adaptor proteins and the actin cytoskeleton in epithelial-to-mesenchymal transition. Cell Communication and Signaling. 19 (1), 67 (2021).
- Hill, Z. B., Perera, B. G., Andrews, S. S., Maly, D. J. Targeting diverse signaling interaction sites allows the rapid generation of bivalent kinase inhibitors. ACS Chemical Biology. 7 (3), 487-495 (2012).
- Wu, S., Fu, L. Tyrosine kinase inhibitors enhanced the efficacy of conventional chemotherapeutic agent in multidrug resistant cancer cells. Molecular Cancer. 17 (1), 25 (2018).
- Ayala-Aguilera, C. C., et al. Small molecule kinase inhibitor drugs (1995-2021): Medical indication, pharmacology, and synthesis. Journal of Medicinal Chemistry. 65 (2), 1047-1131 (2022).
- Lyczek, A., et al. Mutation in Abl kinase with altered drug-binding kinetics indicates a novel mechanism of imatinib resistance. Proceedings of the National Academy of Sciences. 118 (46), 2111451118 (2021).
- Musumeci, F., Schenone, S., Brullo, C., Botta, M. An update on dual Src/Abl inhibitors. Future Medicinal Chemistry. 4 (6), 799-822 (2012).
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update. Pharmacological Research. 152, 104609 (2020).
- Cohen, P., Cross, D., Jänne, P. A. Kinase drug discovery 20 years after imatinib: Progress and future directions. Nature Reviews Drug Discovery. 20 (7), 551-569 (2021).
- Feng, S., Chen, J. K., Yu, H., Simon, J. A., Schreiber, S. L. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science. 266 (5188), 1241-1247 (1994).
- Alexandropoulos, K., Cheng, G., Baltimore, D. Proline-rich sequences that bind to Src homology 3 domains with individual specificities. Proceedings of the National Academy of Sciences. 92 (8), 3110-3114 (1995).
- Feng, S., Kasahara, C., Rickles, R. J., Schreiber, S. L. Specific interactions outside the proline-rich core of two classes of Src homology 3 ligands. Proceedings of the National Academy of Sciences. 92 (26), 12408-12415 (1995).
- Polverini, E., Rangaraj, G., Libich, D. S., Boggs, J. M., Harauz, G. Binding of the proline-rich segment of myelin basic protein to SH3 domains: Spectroscopic, microarray, and modeling studies of ligand conformation and effects of posttranslational modifications. Bioquímica. 47 (1), 267-282 (2008).
- Weng, Z., et al. Structure-function analysis of SH3 domains: SH3 binding specificity altered by single amino acid substitutions. Molecular and Cellular Biology. 15 (10), 5627-5634 (1995).
- Mandal, D., Nasrolahi Shirazi, A., Parang, K. Cell-penetrating homochiral cyclic peptides as nuclear-targeting molecular transporters. Angewandte Chemie. 50 (41), 9633-9637 (2011).
- Hussein, W. M., Skwarczynski, M., Toth, I. . Peptide Synthesis: Methods and Protocols. , (2020).
- Chhikara, B. S., et al. Phenylpyrazalopyrimidines as tyrosine kinase inhibitors: Synthesis, antiproliferative activity, and molecular simulations. Molecules. 25 (9), 2135 (2020).
- TRANSCREENER ADP2 Fl Assay. BellBrook Labs Available from: https://www.bellbrooklabs.com/wp-content/uploads/2021/03/Tech-Manual-ADP2-Fl-v031621.pdf (2022)
- Sanner, M. F., et al. Cyclic peptides as protein kinase inhibitors: Structure-activity relationship and molecular modeling. Journal of Chemical Information and Modeling. 61 (6), 3015-3026 (2021).
- Nahhas, A. F., Nahhas, A. F., Webster, T. J. The physical properties of tripeptide stereocomplex nano-formations. Journal of Biomedical Nanotechnology. 16 (10), 1495-1503 (2020).
- Nahhas, A. F., Chang, R., Webster, T. J. Introducing unnatural amino acids-containing tripeptides as antimicrobial and anticancer agents. Journal of Biomedical Nanotechnology. 14 (5), 987-993 (2018).
- Deng, G., et al. Tryptophan-containing dipeptide derivatives as potent PPARγ antagonists: design, synthesis, biological evaluation, and molecular modeling. European Journal of Medicinal Chemistry. 43 (12), 2699-2716 (2008).
- Chetty, V. T., Sharma, A. M. Can PPARγ agonists have a role in the management of obesity-related hypertension. Vascular Pharmacology. 45 (1), 46-53 (2006).
- Skeggs, L. T., Kahn, J. R., Shumway, N. P. The preparation and function of the hypertensin-converting enzyme. Journal of Experimental Medicine. 103 (3), 295-299 (1956).
- Lunow, D., Kaiser, S., Brückner, S., Gotsch, A., Henle, T. Selective release of ACE-inhibiting tryptophan-containing dipeptides from food proteins by enzymatic hydrolysis. European Food Research and Technology. 237 (1), 27-37 (2013).
- Weber, J., Wilke-Mounts, S., Grell, E., Senior, A. E. Tryptophan fluorescence provides a direct probe of nucleotide binding in the noncatalytic sites of Escherichia coli F1-ATPase. The Journal of Biological Chemistry. 269 (15), 11261-11268 (1994).
- Iavarone, A. T., Patriksson, A., vander Spoel, D., Parks, J. H. Fluorescence probe of Trp-cage protein conformation in solution and in gas phase. Journal of the American Chemical Society. 129 (21), 6726-6735 (2007).
- Nongonierma, A. B., Fitzgerald, R. J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by tryptophan containing dipeptides. Food & Function. 4 (12), 1843-1849 (2013).
- Bjelke, J. R., et al. Dipeptidyl peptidases 8 and 9: specificity and molecular characterization compared with dipeptidyl peptidase IV. The Biochemical Journal. 396 (2), 391-399 (2006).

.
