A Rapid Strategy for the Isolation of New Faustoviruses from Environmental Samples Using Vermamoeba vermiformis
Summary
We describe here the latest advances in viral isolation for the characterization of new genotypes of Faustovirus, a new asfarvirus-related lineage of giant viruses. This protocol can be applied to the high throughput isolation of viruses, especially giant viruses infecting amoeba.
Abstract
The isolation of giant viruses is of great interest in this new era of virology, especially since these giant viruses are related to protists. Giant viruses may be potentially pathogenic for many species of protists. They belong to the recently described order of Megavirales. The new lineage Faustovirus that has been isolated from sewage samples is distantly related to the mammalian pathogen African swine fever virus. This virus is also specific to its amoebal host, Vermamoeba vermiformis, a protist common in health care water systems. It is crucial to continue isolating new Faustovirus genotypes in order to enlarge its genotype collection and study its pan-genome. We developed new strategies for the isolation of additional strains by improving the use of antibiotic and antifungal combinations in order to avoid bacterial and fungal contaminations of the amoeba co-culture and favoring the virus multiplication. We also implemented a new starvation medium to maintain V. vermiformis in optimal conditions for viruses co-culture. Finally, we used flow cytometry rather than microscopic observation, which is time-consuming, to detect the cytopathogenic effect. We obtained two isolates from sewage samples, proving the efficiency of this method and thus widening the collection of Faustoviruses, to better understand their environment, host specificity and genetic content.
Introduction
The discovery of giant viruses, especially those belonging to the Megavirales order, completely changed the world of viruses in terms of particle size and genome complexity. Viruses were previously thought to be small entities, and the Mimivirus appeared to break all the rules.1 Metagenomic data suggests the ubiquity of giant viruses not only in the environment, 2-5 but also in humans.6 Therefore, there is still a need to search for these viruses on a large scale. The diversity of these giant viruses was assessed by sampling not only a variety of aquatic environments and their associated sediments worldwide,7-11 but also by screening a variety of human samples12,13 and environmental samples.7,9 The Acanthamoeba polyphaga mimivirus was isolated by co-culture using phagocytic protists, primarily Acanthamoeba spp.14-16 An entire collection of giant viruses were then also isolated from this specified protist host, which made the scientific community restrict its research and isolation procedure for Acanthamoeba spp. Clearly this reliance on a single host species has resulted in a large fraction of viruses being overlooked. The fact that the giant virus, CroV, was isolated with the highly motile marine protozoa Cafeteria roenbergensis,17,18 demonstrates the need to use a wider range of protozoa in order to discover new lineages or families of giant viruses. Reteno et al. managed to select other protozoa as cell hosts which had never previously been used, and isolated the new Asfar-related lineage of giant viruses (the newly named Faustovirus).19
In an attempt to isolate new Faustovirus genotypes in order to expand the members of this viral lineage, we modified our isolation procedures and used them to screen environmental samples capable of harvesting new Faustoviruses. We then described the entire protocol to characterize the new isolates. We assessed Vermamoeba vermiformis, the most common free-living protist found in human environments,20-22 which is already used in the isolation of the first Faustovirus prototype E12.19 This protist is currently still host-specific for Faustovirus. We knew that none of the known giant viruses was pathogenic for this amoeba, because no attempts to grow other giant viruses in our lab showed amoeba lysis or viral growth. For this reason, we believe that V. vermiformis is the best and most unique cell support known to isolate new Faustoviruses.
Protocol
1. Sample Collection
- Collect 70 samples from different environments and regions. In this case, use the following: 5 dirty water samples from the village of Saint Pierre de Meyzoargues (France), 15 samples from the lake in Parc Borély in Marseille (France); 15 sea water samples with sediment from the rocky inlets at Samena in Marseille (France), 25 river water samples from the Alps (France), and finally, 10 samples from sewage in La Ciotat (France).
- Vortex samples for homogenization before inoculating, for one minute at room temperature without any treatment.
2. Isolation Procedure
- Host Preparation for Blind Culture Procedures and Sample Inoculation
- Use V. vermiformis (strain CDC19) as a cell support for the co-culture.
- Maintain the amoebae at 28 °C, in a 75 cm2 cell culture flask with 30 ml of protease-peptone-yeast extract-glucose medium (PYG). Please check PYG composition in Table 1.
Note: After 48 hr, amoebae are quantified by a normal count using counting slides (e.g., Kovaslides). - Harvest cells at a concentration of 5 x 105 to 106 cell/ml, and pellet amoebae by centrifugation at 720 x g for 10 min.
- Remove the supernatant by aspiration, and re-suspend the amoebae pellet in 30 ml of sterile Page's amoebal saline (PAS) Table 1. Repeat this rinsing procedure.
- Re-suspend amoebae in 30 ml of starvation medium with an antibiotic and antifungal mixture at a concentration of approximately 106 amoeba/ml. The starvation medium is a survival medium for the amoeba, enabling it to stay alive without encystment or multiplication. Check composition in Table 1.
Note: The antimicrobial agent mix contained 10 µg/ml vancomycin, 10 µg/ml imipenem, 20 µg/ml ciprofloxacin, 20 µg/ml doxycycline, and 20 µg/ml voriconazole. This mix served to reduce the bacterial and fungal contamination that can decrease or alter viral multiplication. - Directly inoculate 25 µl of each sample onto the 200 µl amoeba monolayer in a 96-well plate flat bottom favoring the amoeba adherence and incubate at 30 °C in a humid environment (a humid environment consists of placing a wet compress inside the bag containing the plate in order to avoid evaporation). This is the primo-culture. Leave two wells of amoebae without any sample inoculation; this acts as the negative control. Incubate this primo-culture for three days.
- Three days after the first inoculation, sub-culture the primo-culture plate on fresh amoebae as described above without any microscopic observation. Incubate the new plate for three days under the same conditions as the primo-culture.
- Perform the final culture after these three days of incubation the same way as for the primo- and sub-culture. Incubate the final culture for two days. Then proceed to detection.
- Detection of Lysis by Automated Flow Cytometry
Note: Blind culture coupled with flow cytometry differs from the previous standard system of isolation. It is more sensitive, precise and automated. For detection purposes, we applied a new technique developed to detect lysis at the highest speed and without microscopic observation. This technique is advantageous for screening samples and has better sensitivity than older techniques.7-9- Use the 96-well plate of the final co-culture to detect lysis.
- Turn on the cytometer and run a clean and rinse cycle in order to obtain lower background noise according to manufacturer's protocol.
- Launch the High Throughput Sampler (HTS) combined with flow cytometer to automatically load samples from the 96-well plate and perform a completely automated acquisition within 40 min according to manufacturer's protocol.
- Set up the HTS as follows: sample volume 150 µl, mixing volume 50 µl, mixing speed 180, number of mixes 5, washing volume between each sample 400 µl, flow rate 2.5 µl/sec, number of recorded events 10,000.
- Assess the negative control wells of the final co-culture micro-plate first in order to gate the amoeba population and to determine the percentage of these cell hosts according to their physical characteristics. Be sure to have a homogeneous amoeba population and a second population of a lower events corresponding to debris and noise.
Note: This gating procedure distinguishes subcellular debris and clumps from single cells by size, estimated by forward scatter. It is therefore important to accurately determine the percentage of each population using the best possible gating procedure. These percentages may vary, especially if many types of protozoa are used, so it is important always to have a negative control, which can be used as the reference population for the rest of the samples. - Start the gating by clicking acquire sample.
- Record the number of events not less than 10,000 events.
- Localize the amoeba population of interest using the arrow. This is how to define the gates.
- After gating, let the HTS spot or locate the plate by clicking 'Home', then run the entire plate automatically. Perform data acquisition and analysis according to the size and structure parameters (respectively, FSC (forward scatter) and SSC (side scatter)).
Note: Detecting the loss of the amoeba-gated population signals the presence of a pathogenic agent responsible for amoeba lysis. Protozoa lysis associated with virus infection is detected with an arbitrarily designed threshold of 50% lysis of the amoeba population gated by the analyzer and compared to the same non-infected population used as a reliable negative control.
3. Characterization of the New Isolates after Lysis Detection
- Preliminary Staining to Reveal the Presence of Giant Viruses
- After lysis detection by flow cytometry, pipette the lysed co-cultures to suspend the remaining amoebae. Then directly cytocentrifuge 50 µl of the suspension at 800 x g for 10 min.
- After centrifugation, fix the slides with a pure methanol solution. Stain the slides using a commercial rapid staining of Eosin/blue Azur and DAPI staining and observe under a light microscope at 1,000X magnification in order to check for the presence of virus factories.
- For rapid staining, plunge the slides into the first eosin solution (4 sec repeated three times) then pass directly to plunge slides into the second fixing solution containing blue Azur for 6 sec, and finally rinse the slides for 45 sec with a phosphate buffer solution pH 7.2. Leave the slides to dry at room temperature, then proceed to observation.
- For DAPI staining, deposit 15 µl of the DAPI commercial stock solution onto the fixed dry slide. Protect from light then proceed to observation.
- Electron Microscopy
- After the first identification on the slides, assess the presence of the giant virus through electron microscopy observation of supernatant cultures.
- Deposit 5 µl of the positive sample onto the glow-discharged grid. Wait for approximately 20 min at room temperature.
- Dry the grid carefully and deposit on it a small drop of 1% ammonium molybdate for 10 sec. Leave the grid to dry for 5 min.
- Proceed to electron microscopy at 200 keV.
- Place the grid on the holder; introduce the holder into the microscope. Check the vacuum overview by clicking on the corresponding icon. Close valves and begin observation at a spot size 5, and magnification of x 25,000.
- Measure the giant virus using ImageJ or directly on the microscope using the measure tool of the microscope located on the acquisition screen.
Note: Classify the sample into the Faustovirus group with a capsid size of approximately 200 nm.
- Biologie moléculaire
Note: Following this identification, molecular biology tests were crucial for the confirmation in order to distinguish Faustovirus from Marseillevirus, which has the same capsid size and appearance under electron microscopy, although they do not have the same host specificity. The Marseillevirus cannot grow on Vermamoeba, the Faustovirus is specific to Vermamoeba and all attempts to grow it on other amoeba such as Acanthamoeba have failed to date. The design and application of the specific primer/probe systems listed in the works of Reteno et al. enabled a more precise preliminary classification of the newly isolated viruses through amplification and sequencing of the viral genes.- Extract DNA from the positive culture samples using an automated extraction system according to the manufacturer's protocol.
- Perform Real-time PCR using a thermocycler as described in Reteno et al.19
- Sequence using the same primers that were used for amplification. Use the primers targeting DNA-directed RNA polymerase subunit 1 gene: Fstv_S2F 5'-CCA GGA CAT GAT GGT CAC ATA G-3' (forward) and Fstv_S2R 5'-TTG CAC CTC CGC AGT TAA A-3' (reverse) with the Fstv_S2P FAM-TATGCTCCAATGGCCTTCAACGACA-TAMRA probe.
4. Virus Production, Purification and Genome Sequencing
- Subculture the single virus amoeba culture for end-point dilution cloning.
- For the production: prepare 20 flasks containing 30 ml of PYG, 10 ml of Vermamoeba vermiformis and 5 ml of the isolated virus already transferred from wells to small flasks.
- Incubate at 30 °C until complete lysis of the amoeba. Observe every day by inverted optical microscopy until all amoeba are lysed.
- After complete lysis, pool all flasks into one recipient. Filter with a 0.45 µm filter to eliminate debris.
- Start the purification by ultracentrifugation at 89,800 x g for 75 min. Make sure to calibrate the weight of the tubes before starting centrifugation.
- After centrifugation, remove the supernatant from each tube by aspiration and re-suspend the pellet in PAS with the same volume used for calibration, before repeating centrifugation.
- As above, remove the supernatant and re-suspend all the pellets in 1 ml of phosphate buffered saline (PBS).
- Purify the virus that is produced, using 25% sucrose (27.5 g of sucrose in 100 ml of PBS, filtered on a 0.22 µm filter).
- Use 8 ml of sucrose and 2 ml of the viral suspension. Centrifuge at 89,800 x g for 1 hr 15 min. Re-suspend the pellet in 1 ml of PBS. Store at -80 °C.
Representative Results
The system studied in this manuscript validated its proof of concept by isolating two new Faustoviruses. Of the 70 samples tested, two episodes of lysis were detected, in contrast to our reliable negative controls. The negative control for lysis contained an 86% amoeba population. By contrast, the positive samples (ST1 for Saint Pierre de Meyzoargues), and (LC9 for the La Ciotat Sample 9) showed a dramatic decline in gated amoebae; more than 60% of amoebae were lysed with the highest percentage of debris. These robust results of the detection stage of our method are represented in Figure 1. Eosin/blue Azur and DAPI staining confirmed the presence of virus factories inside the amoebae, shown in Figure 2. Electron microscopy revealed the typical appearance of Megavirales with an icosahedral capsid, 200 nm in size, and lacking fibrils (Figure 3). A specific PCR for giant viruses, particularly for Faustovirus, confirmed our previous findings. It must be checked by PCR in order to eliminate ambiguous results or contaminated isolations. Viruses were cloned, produced and purified for whole genome sequencing. The genomes of the newly isolated Faustoviruses are submitted under the following Bioproject: PRJEB11169. The primo-, sub-, and final-culture showed no fungal or bacterial contamination, favoring this viral multiplication. Vermamoeba vermiformis tolerated the anti-fungal and antibiotic mixture. This was clear in the negative control of amoebae where after three days, the final culture contained more than 80% living amoeba. Vermamoeba also provided a good cell host for isolation, producing a significant viral load after lysis.
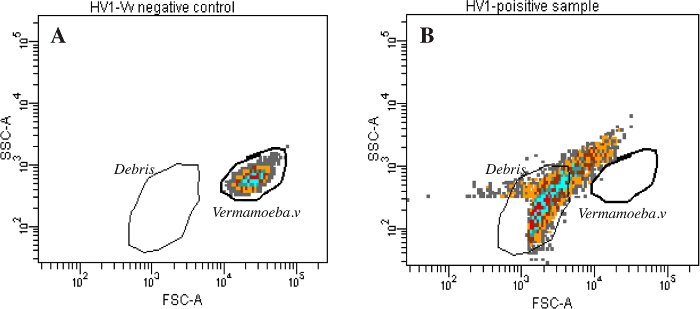
Figure 1: Lysis detection by automated flow cytometry. The data represent the two samples, which harvested Faustovirus and showed amoeba lysis. A living amoeba population was used as a negative control. Two gates were designed in the FSC-SSC plot, potentially corresponding to the cells and debris. After the final co-culture, a large population of debris demonstrated the presence of a potential infection in the samples (ST1 and LC9) (B), compared to the negative control which showed no substantial changes in the gated populations (A). Please click here to view a larger version of this figure.
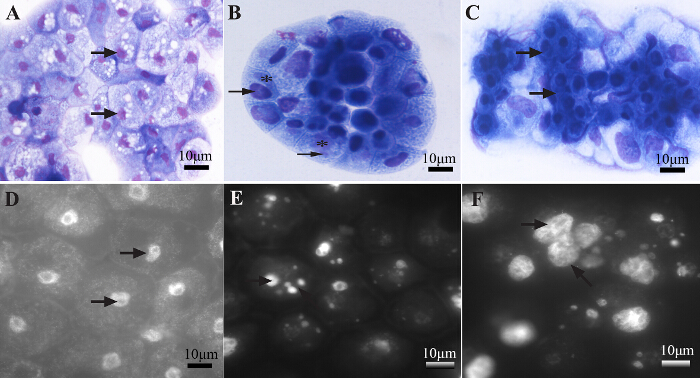
Figure 2: Virus factories typical for some giant viruses in Eosin/blue Azur staining and DAPI staining. Negative control showing the V. vermiformis nucleus using Eosin/blue Azur staining (A), and DAPI staining (D) (arrows point to the nucleus of the amoeba). Magnification at 1,000X. (B, C): Eosin/blue Azur staining at 1,000X magnification of V. vermiformis infected with Faustovirus LC9. Arrows point to the nucleus of the circularized infected amoeba, virus factories are marked with an asterisk. (E, F) DAPI staining at 1,000X magnification of V. vermiformis infected with Faustovirus LC9. Arrows point to virus factories at 8 hr post-infection (E), and 12 hr post-infection (F). Scale bar 10 µm. Please click here to view a larger version of this figure.
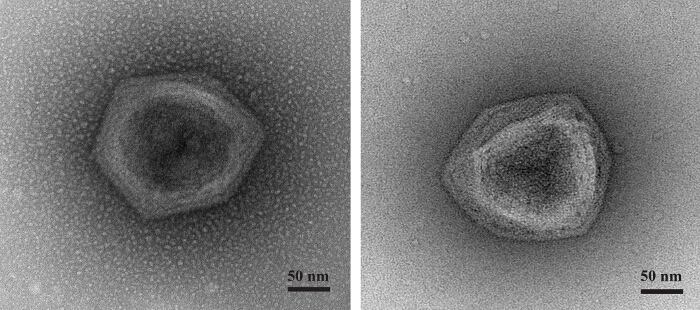
Figure 3: Negative staining micrograph. Negative staining of the viral suspension after lysis detection, showing the newly detected Faustovirus with the typical appearance of Megavirales, with an icosahedral capsid and a total size of 200 nm. Please click here to view a larger version of this figure.
| PYG composition | quantities |
| Proteose peptone | 20 g |
| Yeast extract | 2 g |
| MgSO4. 7H2O | 0.980 g |
| CaCl2 | 0.059 g |
| citrate sodium. Dihydrate | 1 g |
| Fe(NH4) 2(SO4) 2 x 6 H2O | 0.02 g |
| Glucose | 18 g |
| distilled water for | 1 L |
| Adjust pH at 6.8 with HCl or KOH. | |
| Autoclave 15 min at 121 °C. | |
| Starvation medium | quantities |
| Yeast extract | 2 g |
| Glucose | 18 g |
| Fe(NH4) 2(SO4) 2 . 6 H2O | 0.02 g |
| PAS (detailed below) | 1 L |
| Filtered on 0.22 mm | |
| PAS solution A | quantities |
| KH2PO4 | 0.136 g |
| Na2HPO4 | 0.142 g |
| PAS solution B | quantities |
| MgSO4.7H2O | 4.0 mg |
| CaCl2.2H2O | 4.0 mg |
| NaCl | 0.120 g |
| 10 ml of each solution A and B, are added into 1 L of distilled water. | |
Table 1: Solution recipes.
Discussion
The possibility that Faustovirus could be the first member of a new Megavirales family close to ASFV was first suggested by Reteno et al.,19 but some differences can still be distinguished. It appears unclear whether Faustovirus should join the Asfarviridae family or whether it should instead form a new putative viral family. This issue will require further investigation, in particular a more comprehensive characterization of its morphology, host range, replication cycle and gene repertoire. More Faustovirus genotypes should be isolated in order to expand the pan-genome and to better understand the origins and relatives of this viral group or family. The technique we used allowed us to continue isolating new members of this potential new family. This technique is coupled with the slightly modified co-culture technique, which acts as an enrichment step for better detection. The time required by flow cytometry for the acquisition of data for hundreds of samples does not exceed half an hour, which is a major benefit compared to the previous standard co-culture system.9 The technique is far better than the previous standard system, particularly in terms of its higher sensitivity and precise quantification.
Of the 70 samples launched, we isolated two further Faustoviruses from sewage, which could provide information about the environmental specificity or ecosystem of this virus. Indeed, the specificity of Faustovirus for the protist Vermamoeba vermiformis,19 as well as the new classification of HcDNAV among the Asfarviridae23 and the fact that this virus infects many strains of dinoflagellate Heterocapsa spp but is incapable of infecting other types of phytoplanktons24 suggests the presence of host-specificity among Asfarvirus or its close relatives.
These findings imply the use of new cellular supports acting as specific or non-specific hosts, which can harbor these kinds of viruses. Our technique can be adapted to other types of protists in different conditions. Our method marks progress in terms of viral isolation and culture techniques. In summary, enrichment steps with the most appropriate antibacterial and antifungal mixture are crucial for the elimination of any possible bacterial or fungal contamination. Our starvation medium was the best solution to maintaining the amoeba in the best conditions for co-culture, while the PAS medium used in routine culture appears to be inappropriate for Vermamoeba spp, because fast encystment was observed after 24 hr of incubation. This was a big step towards optimal culture conditions where previous techniques had thus far failed. In order to obtain the best results using the culture protocols described here, it is recommended to use fresh amoebae capable of phagocytosis. The antibiotic and antifungal mixture should be non-toxic for protists; hence, it is recommended to test the viability of the used protozoon on the mixture. All used materials should be sterilized. The work should be conducted in a microbiological secured post class II to avoid any likely contamination. Incubation times and the three stages of culture should be respected as described. The processing time for flow cytometry detection can be calibrated depending on the organisms studied. A small shift in the population from the original gating may be observed, but this depends on the reliable negative control used as a reference population.
Multi-resistant bacteria that can be found in culture and are pathogens for amoeba can somehow limit our viral isolation method, where we can have bacterial growth masking the viruses' multiplication. Varying the antibiotic mixture or even filtrating the culture in order to eliminate the bacteria of more than 200 nm sized should manage this limitation sometimes.
All these adapted conditions offer a new optimal isolation procedure, which supports viral multiplication. This technique presents a considerable advantage in the discovery of new viruses, particularly Megavirales, to better understand their diversity, origins, and potential pathogenicity.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements to make.
Materials
| LSR FORTESSA cytometer | BD Biosciences | France | 649225B4 |
| TECNAI G2 F20 | FEI | Germany | 5027/11 |
| Optical inverted microscope | leica | France | 72643 |
| DNA extraction | Qiagen EZ1 Advanced XL Extraction Robot | France | L106A0452 |
| PCR Cycler CFX96 | Bio rad | France | 785BR06298 |
| PYG medium , PAS, Starvation medium | In house laboratory production | Marseille URMITE | x |
| Amoeba strain CDC-19 | ATCC | France | 50237 |
| Plates | Cellstar | France | 655180 |
| PCR materials, primers. | eurogentec | France | Primers cited in manuscript |
| glasstic slide 10 with grids | Kova | USA | H899871441F |
| Eosin/ blue Azur-Hemacolor stain | Merck milipore | France | 111955,6,57,109468 |
| Vacuum driven filters | Thermo scientific | France | BPV4550 / 20170115 |
| Phosphate-Buffered Saline | Thermo Fisher scientific | France | 10010-023 |
| DAPI stain | Life Technologies | France | D1306 |
| cytospin 4 cytocentrifuge | Thermo Fisher scientific | France | 10522013JT184-31 |
| Single cytology tunnel | Biomedical polymers inc. | France | BMP-cyto-S50 |
| Carbon grids | Euromedex | France | FCF400NI |
| Ammonium molibdate | VWR internationanl | France | 21276185 |
| Flasks | SARSTEDT | Germany | 833911 |
| 0.22μm filters | Milex millipor | France | SE2M229104 |
| Ultracentrifuge Sorval WX 80 | Thermo scientific | France | 9102448 |
| Rapid-flow filters | Nalgene | France | 450-0020 |
References
- Raoult, D., et al. The 1.2-megabase genome sequence of Mimivirus. Science. 306 (5700), 1344-1350 (2004).
- Claverie, J. -. M. Giant viruses in the oceans: the 4th Algal Virus Workshop. Virol J. 2, 52-52 (2004).
- Monier, A. A., et al. Marine mimivirus relatives are probably large algal viruses. Audio, Transactions of the IRE Professional Group on. 5, 12-12 (2007).
- Monier, A., Claverie, J. M., Ogata, H. Taxonomic distribution of large DNA viruses in the sea. Genome Biol. , (2008).
- Claverie, J. -. M., et al. Mimivirus and Mimiviridae: Giant viruses with an increasing number of potential hosts, including corals and sponges. J Invertebr Pathol. 101 (3), 9-9 (2009).
- Colson, P., et al. Evidence of the megavirome in humans. J Clin Virol. 57 (3), 191-200 (2013).
- La Scola, B., et al. Tentative characterization of new environmental giant viruses by MALDI-TOF mass spectrometry. Intervirology. 53 (5), 344-353 (2010).
- Boughalmi, M., et al. High-throughput isolation of giant viruses of the Mimiviridae and Marseilleviridae families in the Tunisian environment. Environ Microbiol. , (2012).
- Pagnier, I., et al. A decade of improvements in mimiviridae and marseilleviridae isolation from amoeba. Intervirology. 56 (6), 354-363 (2012).
- Philippe, N., et al. Pandoraviruses: Amoeba Viruses with Genomes Up to 2.5 Mb Reaching That of Parasitic Eukaryotes. Science. 341 (6143), 281-286 (2013).
- Legendre, M., et al. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology. PNAS. , (2014).
- Saadi, H., et al. First Isolation of Mimivirus in a Patient With Pneumonia. Clin Infect Dis. , (2013).
- Saadi, H., et al. Shan virus: a new mimivirus isolated from the stool of a Tunisian patient with pneumonia. Intervirology. 56 (6), 424-429 (2013).
- Claverie, J. -. M., Abergel, C. Mimivirus: the emerging paradox of quasi-autonomous viruses. Trends Genet : TIG. 26 (10), 431-437 (2010).
- Colson, P., et al. Viruses with more than 1,000 genes: Mamavirus, a new Acanthamoeba polyphaga mimivirus strain, and reannotation of Mimivirus genes. Genome Biol Evol. 3, 737-742 (2010).
- Claverie, J. -. M., Abergel, C. Open questions about giant viruses. Adv Virus Res. 85, 25-56 (2013).
- Fischer, M. G. M., Allen, M. J. M., Wilson, W. H. W., Suttle, C. A. C. Giant virus with a remarkable complement of genes infects marine zooplankton. PNAS. 107 (45), 19508-19513 (2010).
- Van Etten, J. L. Another really, really big virus. Viruses. 3 (1), 32-46 (2011).
- Reteno, D. G., et al. Faustovirus, an asfarvirus-related new lineage of giant viruses infecting amoebae. J.Virol. , (2015).
- Coşkun, K. A., Ozçelik, S., Tutar, L., Elaldı, N., Tutar, Y. Isolation and identification of free-living amoebae from tap water in Sivas, Turkey. Biomed res Int. 2013, 675145 (2013).
- Bradbury, R. S. Free-living amoebae recovered from human stool samples in Strongyloides agar culture. J Clin microbiol. 52 (2), 699-700 (2014).
- Pagnier, I., Valles, C., Raoult, D., La Scola, B. Isolation of Vermamoeba vermiformis and associated bacteria in hospital water. Microb Pathog. 80, 14-20 (2015).
- Ogata, H., Toyoda, K., et al. Remarkable sequence similarity between the dinoflagellate-infecting marine girus and the terrestrial pathogen African swine fever virus. Virol J. 6, 178-178 (2008).
- Tarutani, K., Nagasaki, K., Itakura, S. Isolation of a virus infecting the novel shellfish-killing dinoflagellate Heterocapsa circularisquama. Aquat Microb Ecol. 23, 103-111 (2001).

